Pharmacokinetics of progesterone
The pharmacokinetics of progesterone, concerns the pharmacodynamics, pharmacokinetics, and various routes of administration of progesterone.[16][17]
 | |
| Clinical data | |
|---|---|
| Routes of administration | • By mouth (capsule) • Sublingual (tablet) • Topical (cream, gel) • Vaginal (capsule, tablet (insert), gel, suppository, ring) • Rectal (suppository) • IM injection (oil solution) • SC injection (aq. soln.) • Intrauterine (IUD) |
| Drug class | Progestogen; Antimineralocorticoid; Neurosteroid |
| Pharmacokinetic data | |
| Bioavailability | Oral: <2.4%[1] Vaginal: 4–8%[2][3][4] |
| Protein binding | 98–99%:[5][6] • Albumin: 80% • CBG: 18% • SHBG: <1% • Free: 1–2% |
| Metabolism | Mainly liver: • 5α- and 5β-reductase • 3α- and 3β-HSD • 20α- and 20β-HSD • Conjugation • 17α-Hydroxylase • 21-Hydroxylase • CYPs (e.g., CYP3A4) |
| Metabolites | • Dihydroprogesterones • Pregnanolones • Pregnanediols • 20α-Hydroxyprogesterone • 17α-Hydroxyprogesterone • Pregnanetriols • 11-Deoxycorticosterone (And glucuronide/sulfate conjugates) |
| Elimination half-life | • Oral: 5 hours (with food)[7] • Sublingual: 6–7 hours[8] • Vaginal: 14–50 hours[9][8] • Topical: 30–40 hours[10] • IM: 20–28 hours[11][9][12] • SC: 13–18 hours[12] • IV: 3–90 minutes[13] |
| Excretion | Bile and urine[14][15] |
Progesterone is a naturally occurring and bioidentical progestogen, or an agonist of the progesterone receptor, the biological target of progestogens like endogenous progesterone.[16] Progesterone also has antimineralocorticoid and inhibitory neurosteroid activity, whereas it appears to have little or no glucocorticoid or antiandrogenic activity and has no androgenic activity.[16] Because of its progestogenic activity, progesterone has functional antiestrogenic effects in certain tissues such as the uterus, cervix, and vagina.[16] In addition, progesterone has antigonadotropic effects due to its progestogenic activity and can inhibit fertility and suppress sex hormone production.[16] Progesterone differs from progestins (synthetic progestogens) like medroxyprogesterone acetate and norethisterone, with implications for pharmacodynamics and pharmacokinetics as well as efficacy, tolerability, and safety.[16]
Progesterone can be taken by mouth, in through the vagina, and by injection into muscle or fat, among other routes.[16] A progesterone vaginal ring and progesterone intrauterine device are also available as pharmaceutical products.[18][19]
Routes of administration
The pharmacokinetics of progesterone are dependent on its route of administration. The medication is approved in the form of oil-filled capsules containing micronized progesterone for oral administration, termed "oral micronized progesterone" ("OMP") or simply "oral progesterone".[20] It is also available in the form of vaginal or rectal suppositories or pessaries, topical creams and gels,[21] oil solutions for intramuscular injection, and aqueous solutions for subcutaneous injection.[20][12][22]
Routes of administration that progesterone has been used by include oral, intranasal, transdermal/topical, vaginal, rectal, intramuscular, subcutaneous, and intravenous injection.[12] Oral progesterone has been found to be inferior to vaginal and intramuscular progesterone in terms of absorption (low) and clearance rate (rapid).[12] Vaginal progesterone is available in the forms of progesterone gel, rings, and suppositories or pessaries.[12] Advantages of intravaginal progesterone over oral administration include high bioavailability, rapid absorption, avoidance of first-pass metabolism, sustained plasma concentrations, and a local endometrial effect, while advantages of intravaginal progesterone relative to intramuscular injection include greater convenience and lack of injection site pain.[12]
Intranasal progesterone as a nasal spray has been found to be effective in achieving therapeutic levels, and was not associated with nasal irritation, but was associated with an unpleasant taste of the spray.[12] Rectal, intramuscular, and intravenous routes may be inconvenient, especially for long-term treatment.[12] Plasma levels of progesterone are similar after vaginal and rectal administration in spite of the different routes of administration, and rectal administration is an alternative to vaginal progesterone in conditions of vaginal infection, cystitis, recent childbirth, or when barrier contraception methods are used.[12] Intramuscular injection of progesterone may achieve much higher levels of progesterone than normal luteal phase concentrations and levels achieved with other routes.[12]
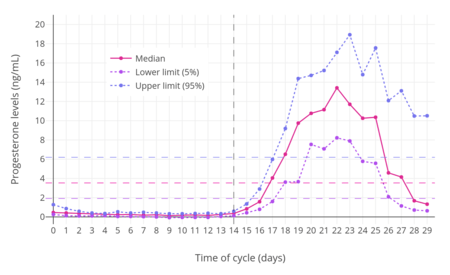
For purposes of comparison with normal physiological circumstances, luteal phase levels of progesterone are 4 to 30 ng/mL (with levels of 5 to 9 ng/mL during the mid-luteal phase), while follicular phase levels of progesterone are 0.02 to 0.9 ng/mL, menopausal levels are 0.03 to 0.3 ng/mL, and levels of progesterone in men are 0.12 to 0.3 ng/mL.[24][25] During pregnancy, levels of progesterone in the first 4 to 8 weeks are 25 to 75 ng/mL, and levels are typically around 140 to 200 ng/mL at term.[26][24] Production of progesterone in the body in late pregnancy is approximately 250 mg per day, 90% of which reaches the maternal circulation.[27]
| Route | Form | Dose | Major brand names | Availability | |
|---|---|---|---|---|---|
| Oral | Capsule | 100, 200, 300 mg | Prometrium, Utrogestan, Microgest | Widespread | |
| Sublingual | Tablet | 10, 25, 50, 100 mg | Luteina, Lingusorbs, Membrettes | Poland and Ukraine | |
| Topicala | Gel | 1% (25 mg) | Progestogel | Limited (mostly Europe) | |
| Vaginal | Capsule | 100, 200 mg | Utrogestan | Widespread | |
| Tablet | 100 mg | Endometrin, Lutinus | Widespread | ||
| Gel | 4%, 8% (45, 90 mg) | Crinone, Crinone 8%, Prochieve | Widespread | ||
| Suppository | 200, 400 mg | Cyclogest | Limited (mostly Europe) | ||
| Ring | 10 mg/day for 3 months | Fertiring, Progering | Chile, Ecuador, and Peru | ||
| Rectal | Suppository | 200, 400 mg | Cyclogest | Limited (mostly Europe) | |
| IM injection | Oil solution | 5, 10, 25, 50, 100 mg/mL | Progestaject, Gestone, Strone | Widespread | |
| Aqueous suspension | 12.5, 30, 100 mg/mL | Agolutin | Czech Republic and Slovakia | ||
| SC injection | Aqueous solution | 25 mg/vial | Prolutex | Limited (some Europe) | |
| Intrauterine | IUD | 38 mg | Progestasert | Discontinued | |
| Footnotes: a = For local application to the breasts; negligible systemic effect. Notes: (1): This table does not include combination products, such as progesterone in combination with estrogens. (2): Some of these formulations have been marketed previously but may no longer be available. (3): The availability of pharmaceutical progesterone products differs by country (see Progesterone (medication) § Availability). (4): This table does not include compounded progesterone products. Sources: See template. | |||||
| Route | Dose | Time | P4 (levels) | Allo (levels) | Preg (levels) | Assay | |
|---|---|---|---|---|---|---|---|
| Oral (in oil, micronized) | 100 mg 100 mg 100 mg 200 mg 200 mg 200 mg 300 mg 300 mg 600 mg 1,200 mg | 2.7 hours ? ? 2.2 hours ? ? 2.0 hours ? ? ? | 10.2 ng/mL 1.5–6.5 ng/mL 1.5–2.2 ng/mL 19.9 ng/mL 3.2–13.8 ng/mL 12 ng/mL 49.8 ng/mL 9.0–32.2 ng/mL 32.8 ng/mL 58.5 ng/mL | ND ND 14 ng/mL ND ND 30 ng/mL ND ND ND ND | ND ND 3.6 ng/mL ND ND 60 ng/mL ND ND ND ND | RIA ? LC–MS RIA ? RIA (+CS) RIA RIA RIA RIA | |
| Vaginal | 25 mg 50 mg 100 mg 100 mg 200 mg 400 mg | ? ? ? ? 12 hours ? | 7.3 ng/mL 8.8 ng/mL 9.5–19.0 ng/mL 5 ng/mL 5.2 ng/mL 16 ng/mL | ND ND ND 3.5 ng/mL ND 1.2 ng/mL | ND ND ND NC ND 0.3 ng/mL | ? ? ? RIA (+CS) RIA RIA (+CS) | |
| Rectal | 25 mg 100 mg 200 mg | ? ? ? | 6.4 ng/mL 22.5 ng/mL 19.3–20.3 ng/mL | ND ND ND | ND ND ND | ? ? ? | |
| Intramuscular (in oil) | 25 mg 50 mg 100 mg 200 mg | ? ? ? ? | 16.9 ng/mL 36.5 ng/mL 81.8–83.8 ng/mL 194–270 ng/mL | ND ND ND ND | ND ND ND ND | ? ? ? ? | |
| Notes: In the case of oral progesterone, RIA may overestimate levels of progesterone by as much as 8-fold due to its low specificity and cross-reactivity with high levels of progesterone metabolites. LC–MS and RIA plus CS are exact methods and can be considered accurate, on the other hand. Sources: See template. | |||||||
| Route | Form | Dose | Cmax | Tmax | t1/2 | AUC0–t | AUC0–∞ |
|---|---|---|---|---|---|---|---|
| Oral | Capsule | 200 mg | 4.3–11.7 ng/mL | 2–2.5 hours | ? | ? | ? |
| Sublingual | Tableta | 100 mg | 13.5 ng/mL | 1–4 hours | ~6–7 hours | ? | ? |
| Suspension | 100 mg | 17.6 ng/mL | 0.5–1 hours | ? | ? | ? | |
| Vaginal | Tableta | 100 mg | 10.9 ng/mL | 6–7 hours | 13.7 hours | ? | ? |
| Capsule | 100 mg | 9.7 ng/mL | 1–3 hours | ? | ? | ? | |
| IM injection | Oil solution | 50 mg | 14.3 ng/mL | 8.7 hours | ? | ? | ? |
| 100 mg | 113 ng/mL | 6.7 hours | 22.3 hours | 2049 ng/mL/h | 2097 ng/mL/h | ||
| Aqueous solutionb | 100 mg | 440 ng/mL | 0.88 hours | 14.3 hours | 1902 ng/mL/h | 1919 ng/mL/h | |
| SC injection | Aqueous solutionb | 25 mg | 57.8 ng/mL | 0.92 hours | 13.1 hours | 338 ng/mL/h | 349 ng/mL/h |
| 50 mg | 103 ng/mL | 0.92 hours | 17.2 hours | 729 ng/mL/h | 746 ng/mL/h | ||
| 100 mg | 235–300 ng/mL | 0.92 hours | 17.2–17.6 hours | 1466–1856 ng/mL/h | 1490–1885 ng/mL/h | ||
| Footnotes: a = Luteina. b = Prolutex (progesterone complexed with β-cyclodextrin to increase water solubility). Sources: See template. | |||||||
| Group | P4 production | P4 levels | ||
|---|---|---|---|---|
| Prepubertal children | ND | 0.06–0.5 ng/mL | ||
| Pubertal girls Tanner stage I (childhood) Tanner stage II (ages 8–12) Tanner stage III (ages 10–13) Tanner stage IV (ages 11–14) Tanner stage V (ages 12–15) Follicular phase (days 1–14) Luteal phase (days 15–28) | ND ND ND ND ND ND | 0.22 (<0.10–0.32) ng/mL 0.30 (0.10–0.51) ng/mL 0.36 (0.10–0.75) ng/mL 1.75 (<0.10–25.0) ng/mL 0.35 (0.13–0.75) ng/mL 2.0–25.0 ng/mL | ||
| Premenopausal women Follicular phase (days 1–14) Luteal phase (days 15–28) Oral contraceptive (anovulatory) | 0.75–5.4 mg/day 15–50 mg/day ND | 0.02–1.2 ng/mL 4–30 ng/mL 0.1–0.3 ng/mL | ||
| Postmenopausal women Oophorectomized women Oophorectomized and adrenalectomized women | ND 1.2 mg/day <0.3 mg/day | 0.03–0.3 ng/mL 0.39 ng/mL ND | ||
| Pregnant women First trimester (weeks 1–12) Second trimester (weeks 13–26) Third trimester (weeks 27–40) Postpartum (at 24 hours) | 55 mg/day 92–100 mg/day 190–563 mg/day ND | 9–75 ng/mL 17–146 ng/mL 55–255 ng/mL 19 ng/mL | ||
| Men | 0.75–3 mg/day | 0.1–0.3 ng/mL | ||
| Notes: Mean levels are given as a single value and ranges are given after in parentheses. Sources: See template. | ||||
Oral administration
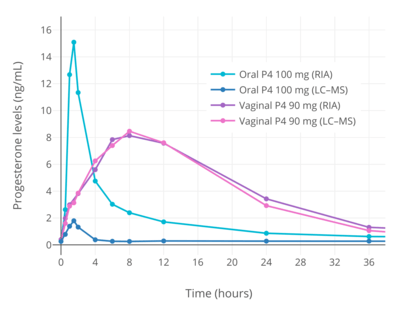
| Parameter | Oral 100 mga | Vaginal 90 mgb | ||
|---|---|---|---|---|
| LC–MS | RIA | LC–MS | RIA | |
| Cmax (ng/mL) | 2.2 | 19.4 | 10.5 | 10.5 |
| Cmax (ng/mL/mg) (DN) | 0.02 | ND | 0.12 | ND |
| Cavg(0–24) (ng/mL) | 0.14 | ND | 5.55 | ND |
| Tmax (h) | 1.0 | 1.0 | 7.7 | 7.7 |
| AUC0–24 (ng•h/mL) | 3.5 | ND | 133.3 | ND |
| AUC0–24 (ng•h/mL/mg) (DN) | 0.035 | ND | 1.5 | ND |
| Abbreviations: DN = Dose-normalized. Footnotes: a = Prometrium. b = Crinone 8% gel. | ||||
Knowledge about the pharmacokinetics of oral progesterone has been complicated by the use of flawed analytical techniques.[29][28][30] When progesterone is taken orally, due to first-pass metabolism, very high levels of its metabolites occur.[29][28][30] Most previous studies have used a method known as radioimmunoassay (RIA) to measure progesterone levels.[29][28][30] However, RIA has high cross-reactivity and is unable to differentiate between progesterone and metabolites such as allopregnanolone and pregnanolone.[29][28][30] As a result, studies that have assessed the pharmacokinetics of oral progesterone using RIA have reported falsely high progesterone levels and inaccurate dependent pharmacokinetic parameters.[29][28][30] Comparative studies using reliable and exact methods such as liquid chromatography–mass spectrometry (LC–MS) and RIA in conjunction with adequate chromatographic separation (CS) have found that RIA overestimates levels of progesterone by 5- to 8-fold.[29][28][30] For this reason, the use of reliable assays is mandatory when studying the pharmacokinetics of oral progesterone, and an awareness of these methodological issues is likewise essential for an accurate understanding of the pharmacokinetics of oral progesterone.[29][28][30] Conversely, the same issues are not applicable to parenteral routes of progesterone such as vaginal administration and intramuscular injection, because these routes are not subject to a first pass and relatively low levels of progesterone metabolites are formed.[29][28][30] Aside from the relevant methodological issues, it is also important to note that when the term "oral progesterone" is used, what is used clinically and what is almost always being referred to, unless noted otherwise, is micronized progesterone suspended in oil.[31][16][29]
The oral bioavailability of progesterone is very low.[31] Studies using RIA have generally measured the bioavailability of oral progesterone as less than 10%,[31] with one study reporting values of 6.2 to 8.6%.[32][11] However, these values are overestimations; a study using LC–MS found that the bioavailability of oral progesterone was only 2.4% relative to vaginal progesterone gel[1] (and notably not relative to the standard of progesterone by intramuscular injection, which has much higher bioavailability than vaginal progesterone).[33][4] The very low bioavailability of oral progesterone is due to the fact that it is poorly absorbed from the gastrointestinal tract and undergoes massive metabolism, resulting in almost complete inactivation during the first pass through the liver.[31][34] Because of its poor oral bioavailability, very high doses of progesterone must be used by the oral route to achieve significant circulating progesterone levels.[31] In addition, oral progesterone is always micronized and suspended in oil.[31][20][33][35] This improves the bioavailability of oral progesterone significantly compared to plain milled progesterone, and allows for it to be used at practical doses.[31]
Micronization is the process of reducing the average diameter of the particles of a solid material.[35] By micronizing progesterone, its particles are made smaller (mainly <10 μM) and its surface area is increased, thereby enhancing absorption from the intestines.[31][35] Suspension and partial solubilization[36] of progesterone in oil containing medium- to long-chain fatty acids likewise improves the bioavailability of oral progesterone.[16][37][38] Progesterone is a lipophilic compound and it has been theorized that suspension of progesterone in oil may improve its absorption by the lymphatic system, thereby allowing a portion of oral progesterone to bypass the first pass through the liver and hence enhancing its bioavailability.[31][39][40][41] Compared to plain milled progesterone, peak levels of progesterone following a single 200 mg oral dose were increased 1.4-fold by micronization, 1.2-fold by suspension in oil, and 3.2-fold by the combination of micronization and suspension in oil.[41] Oral micronized progesterone suspended in oil is rapidly and almost completely absorbed from the intestines.[13] There is wide interindividual variability in the bioavailability of oral progesterone.[16][11] As progesterone was not used orally for many decades due to its poor bioavailability (until the introduction of oral micronized progesterone in oil-filled gelatin capsules in 1980),[33] oral progestins (synthetic progestogens) with improved metabolic stability and high oral bioavailability were developed and have been used clinically instead.[42]
When oral progesterone is used at typical clinical dosages, only very low levels of progesterone are measured using reliable methods.[29][28][30] Following single doses of oral progesterone, peak levels of progesterone of 1.5 to 2.4 ng/mL with 100 mg and 2.8 to 4.7 ng/mL with 200 mg have been measured using LC–MS, liquid chromatography–tandem mass spectrometry (LC–MS/MS), and RIA with adequate CS.[29][43][1] In one such study, although peak levels of progesterone were 2.2 ng/mL after a single 100 mg dose of oral progesterone, levels of progesterone remained significantly elevated for less than about 4 hours, and the average progesterone levels over a period of 24 hours were only 0.14 ng/mL.[30][1] For comparison, normal progesterone levels during the luteal phase of the menstrual cycle with LC–MS/MS are 6.7 to 22.2 ng/mL.[44] When the unreliable method of standalone RIA has been used to measure progesterone levels with oral progesterone, far higher peak levels of 6.5 to 10.2 ng/mL, 13.8 to 19.9 ng/mL, and 32.3 to 49.8 ng/mL have been erroneously observed after single 100, 200, and 300 mg doses, respectively.[32][11] One RIA-based study even reported maximal progesterone levels of 16 to 626 ng/mL (mean 247 ng/mL) with a single 300 mg dose of oral progesterone.[45][46]
Levels of progesterone with oral progesterone have been inaccurately measured by RIA as remaining elevated for 12 to 24 hours.[1][20] Regardless of assay method, peak levels of progesterone following a dose of oral progesterone occur after about 1 to 3 hours.[28] The elimination half-life of progesterone in the circulation is very short at a range of about 3 to 90 minutes.[13] Previous studies using RIA have reported an overestimated elimination half-life of oral progesterone of about 16 to 18 hours.[20] A subsequent, reliable study using high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) reported an elimination half-life of oral progesterone of about 4.6 to 5.2 hours when it was taken with food.[7] Due to the short half-life and duration of action of oral progesterone, it is often taken in divided doses of two or three times per day.[20][47]
Clinical progestogenic potency and effects
Because of studies that used RIA, it was incorrectly believed for many years that oral progesterone could easily achieve luteal phase levels of progesterone or beyond and could produce considerable progestogenic effects.[29][28][30] In actuality, the very low levels of progesterone with oral administration, as measured by reliable methods like LC–MS, appear to be insufficient for adequate and full progestogenic effects.[30][1] This is evidenced by the fact that, in contrast to almost all progestins, an increased risk of endometrial cancer has been observed when oral progesterone is combined with an estrogen in menopausal hormone therapy.[30][1] This finding indicates that typical clinical dosages of oral progesterone are insufficient for full endometrial protection.[30][1] However, in spite of the very low levels of progesterone achieved, typical clinical dosages of oral progesterone do seem to be able to adequately prevent estrogen-induced endometrial hyperplasia.[29][28] On the other hand, oral progesterone fails to produce full endometrial secretory transformation, and is considered to be inappropriate for use in assisted reproduction, whereas vaginal and intramuscular progesterone are effective.[48][49] Even very high doses of 600 mg/day oral progesterone fail to produce full luteal-phase endometrial changes,[50] although doses of 300 to 600 mg/day oral progesterone have reportedly been used for luteal support in assisted reproduction.[49] Research on whether oral non-micronized progesterone has a thermogenic effect has shown conflicting findings in different studies.[51]
The very low levels of progesterone achieved with oral progesterone may also explain the absence of an increase in risk of breast cancer and venous thromboembolism when oral progesterone is added to estrogen therapy in postmenopausal women.[30] Such risks are increased by progestins, which are PR agonists similarly to progesterone, but have not been found to be increased by oral progesterone.[28][30] Since typical clinical dosages of oral progesterone achieve only very low levels of progesterone, and progesterone therapy resulting in adequate progesterone levels has never been properly evaluated in sufficiently large clinical studies, it has been said that notion that progesterone somehow differs from progestins and does not increase the risk of breast cancer or venous thromboembolism are unsubstantiated.[28][30][43] Moreover, it has been said that in the absence of adequate data to the contrary, it would be reasonable to consider progesterone at least equivalent to progestins as a potential risk factor for such complications.[28][30][43] Indeed, the French E3N study did observe a significant increase in risk of breast cancer with estrogen and progesterone therapy in postmenopausal women after long-term (>5-year) administration, which is consistent with a weak proliferative effect of oral progesterone on the breasts such that a longer duration of exposure is necessary for a significant increase in breast cancer incidence to manifest.[28][30]
Influence of food on bioavailability
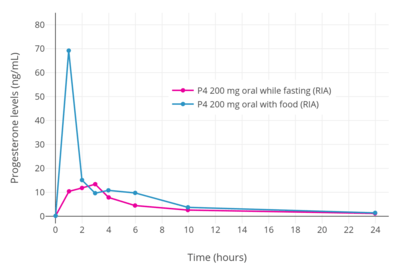
When oral progesterone is taken with food instead of taken in a fasting state, maximal levels of progesterone and overall bioavailability are significantly improved.[11][7] Measured with the unreliable method of RIA, peak levels of progesterone were increased by 5-fold and area-under-the-curve levels of progesterone by 2-fold when oral progesterone was taken with food.[11] In this RIA study, a single dose of 200 mg oral progesterone in fasting and fed conditions resulted in peak levels of progesterone of 13.4 ng/mL and 69.5 ng/mL, respectively, and AUC0–24 levels of progesterone of 91.5 ng/mL and 182.5 ng/mL, respectively.[11]
The improvement in progesterone levels and bioavailability when oral progesterone is taken with food may be due to enhanced lymphatic absorption, allowing oral progesterone to bypass some first-pass metabolism by being taken up by the lymphatic system instead of the liver.[11][31][39][40] In accordance, a related steroid medication, oral testosterone undecanoate in oil-filled capsules, is absorbed by the lymphatic system but must be taken with meals that contain at least a moderate or "normal" amount of fat for adequate bioavailability.[52][53] Intake of oral testosterone undecanoate with food has been found to substantially increase peak testosterone levels and overall bioavailability relative to when the medication is taken in a fasting state.[54][55] Although the bioavailability of oral progesterone is increased if it is taken with food, its overall bioavailability is still relatively low, even if measured using RIA.[17]
First-pass effect and neurosteroids
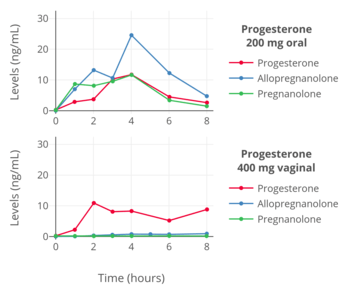
Progesterone is metabolized into allopregnanolone and pregnanolone, which are neurosteroids and potent potentiators of the GABAA receptor.[57][58] The conversion of progesterone into these metabolites is catalyzed by the enzymes 5α- and 5β-reductase and 3α-hydroxysteroid dehydrogenase, and occurs primarily in the liver, but also occurs in reproductive endocrine tissues, the skin, the brain, and other tissues.[59] Due to extensive first-pass metabolism with oral progesterone, about 80 to 90% or more of progesterone is rapidly transformed into these metabolites, and massive quantities of these neurosteroids are consequently formed and circulate throughout the body and brain.[49][60][61][50] It is for this reason that commonly reported side effects of oral progesterone include dizziness, drowsiness, sedation, somnolence, and fatigue.[57][58] Both oral and sufficiently high doses of intramuscular progesterone can produce these sedative effects.[62][63][64] However, compared to oral progesterone, the levels of these neurosteroids have been found to be very low with parenteral routes like vaginal and intramuscular progesterone.[56][63] As with the bioavailability of oral progesterone, there is high interindividual variability in the formation and levels of allopregnanolone and pregnanolone with oral progesterone.[16] As a result, some individuals may experience considerable central depressant effects with oral progesterone, whereas others may experience minimal such effects.[16]
With oral administration of progesterone, allopregnanolone and pregnanolone circulate at higher concentrations than does progesterone.[16][56] These neurosteroid metabolites of progesterone have relatively short biological half-lives in the circulation.[65][66] Because of this, there are dramatic and highly supraphysiological spikes in allopregnanolone and pregnanolone concentrations followed by steep declines with each oral intake of progesterone.[60][61][56] As such, neurosteroid levels fluctuate substantially (e.g., 15-fold in the case of allopregnanolone) and in an unphysiological manner with oral progesterone therapy.[60][50] In addition, consumption of food with oral progesterone increases its absorption by two-fold, and this may also further amplify fluctuations in neurosteroid levels, particularly if food intake with progesterone is not consistent from dose to dose.[11]
In contrast to oral administration, parenteral progesterone, such as with vaginal administration, avoids the first-pass effect, and is not associated with supraphysiological levels of neurosteroid metabolites, nor with spikes or marked fluctuations in neurosteroid levels.[60] Parenteral routes can be used instead of oral administration to avoid adverse effects related to neurosteroid fluctuations if they prove to be problematic.[50][16] Lower doses of oral progesterone (e.g., 100 mg/day) are also associated with relatively reduced formation of neurosteroid metabolites, and may similarly help to alleviate such side effects.[16] In addition, the 5α-reductase inhibitor dutasteride, which blocks the production of allopregnanolone (though not of pregnanolone) from progesterone, has been found to diminish symptoms of premenstrual syndrome.[67]
Pregnenolone, an over-the-counter supplement and close analogue of progesterone, is extensively converted into neurosteroids such as allopregnanolone and pregnanolone with oral administration similarly to progesterone.[68][69][70][71] Conversely, this was not seen with transdermal administration of pregnenolone.[71]
Buccal administration
Progesterone has been studied for use by buccal administration.[16][72][73][74][75][76][77] The medication has been marketed in the form of buccal tablets under the brand names Progesterone Lingusorbs, Lutocylol, Membrettes, and Syngestrets.[78][79] The clinical dosage of buccal progesterone has been described as 10 to 50 mg/day relative to 5 to 60 mg/day in the case of intramuscular injection.[78]
Sublingual administration
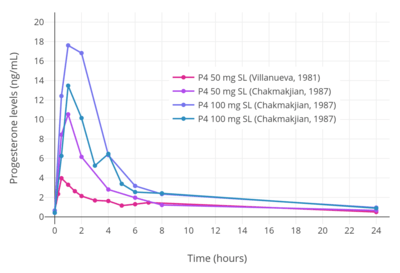
A micronized tablet formulation of progesterone marketed under the brand name Luteina is indicated for use by sublingual administration in addition to the vaginal route and is approved for use in Poland and Ukraine.[82] It is used by the sublingual route at dosages of 50 to 150 mg three to four times per day.[82][8] A single 100 mg sublingual dose of Luteina has been found to reach mean peak progesterone levels of 13.5 ng/mL after 1 to 4 hours, with an elimination half-life of about 6 to 7 hours.[82][8]
A number of other studies have also investigated the use of progesterone by sublingual administration.[83][84][80][81] Older studies have also explored sublingual progesterone.[85][86][87][88] A study of sublingual progesterone for luteal support in patients undergoing embryo transfer found that after sublingual administration of 50 or 100 mg progesterone dissolved in a 1 mL suspension, peak levels of progesterone were reached in 30 to 60 minutes and were on average 17.61 ± 3.78 ng/mL with the 100-mg dose.[83][84] However, the duration was short, with levels of less than 5 ng/mL at 6 hours, and re-administration had to be done two or three times per day for adequate circulating levels of progesterone to be maintained throughout the day.[83][84] Another study found that sublingual progesterone had to be administered at a dose of 400 mg every 8 hours to achieve circulating levels similar to those produced by 100 mg/day intramuscular progesterone.[83] One study administered 400 mg sublingual progesterone three times per day and achieved mean progesterone levels of 57.8 ± 37.4 ng/mL, which were similar to those produced by 50 mg/day intramuscular progesterone.[84]
Intranasal administration
Progesterone has been evaluated by the intranasal route, in the form of a nasal spray, in one study.[83][16][89][90]
Transdermal administration
Progesterone for transdermal or topical administration is not approved by the FDA in the United States.[91][92][43] Multiple pharmaceutical companies have pursued the development of systemic transdermal progesterone formulations, but ultimately none have successfully been developed and introduced for clinical use.[93] Although no formulations of transdermal progesterone are approved for systemic use, a 1% topical gel formulation of progesterone is approved in various countries under the brand name Progestogel for local use on the breasts to treat breast pain.[94][33][95]
Although no formulations of transdermal progesterone are approved for systemic use, topical progesterone is available in the forms of creams and gels from custom compounding pharmacies in some countries, and is also available over-the-counter without a prescription in the United States.[91][92][43] Topical progesterone has been used by thousands of women as a component of menopausal hormone therapy in the United States and Europe.[91] However, these products are unregulated and have not been clinically tested, often with little being known about their pharmacokinetics.[91] In addition, the absorption of topical progesterone may differ significantly from formulation to formulation due to widely varying ingredients.[92] Moreover, the systemic effectiveness of topical progesterone in producing therapeutic progestogenic effects, most importantly adequate endometrial protection against estrogens, is controversial.[91][92]
Some unregulated topical progesterone products contain "wild yam extract" derived from Dioscorea villosa, but there is no evidence that the human body can convert its active ingredient (diosgenin, the plant steroid that is chemically converted to produce progesterone industrially)[96] into progesterone.[97][98]
Absorption and distribution
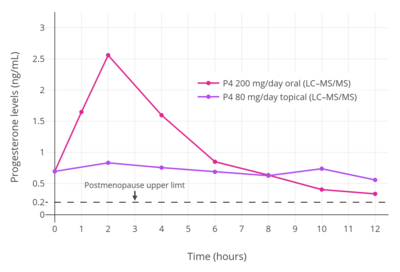
Skin permeability of a compound is based on its physicochemical properties, particularly lipophilicity and hydrophilicity.[16][99] In general, the more polar groups, for instance hydroxyl groups, that are present in a steroid, and hence the more hydrophilic and less lipophilic it is, the lower its skin permeability.[16][99] For this reason, progesterone and estrone have higher skin permeability, estradiol has moderate skin permeability, and estriol and cortisol have lower skin permeability.[16] The transdermal bioavailability of progesterone applied to the breasts is approximately 10%,[93][100][101][102] which is roughly the same as that of the general transdermal absorption of estradiol and testosterone.[93][103][104][105] However, whereas estradiol circulates at levels in the picomolar range (pg/mL), progesterone circulates at concentrations in the nanomolar range (ng/mL), and a relatively large dose is required to produce these levels.[106] The body synthesizes about 25 mg progesterone per day on average during the luteal phase.[17][83] This relatively large quantity by weight has been claimed to require around 50% of the body to be used as a surface of absorption to deliver a similar quantity of progesterone on the basis of its absorption mechanics.[17][83] As such, the transdermal route cannot easily achieve adequate circulating progesterone levels, and this makes transdermal progesterone impractical for systemic therapy.[17][83][106] Clinical studies have found only very low circulating levels of progesterone with the use of topical progesterone, and these levels are thought to be insufficient to confer endometrial protection against estrogens.[91][92] The range of circulating levels of progesterone that has been observed in clinical studies with various formulations and doses of topical progesterone is 0.38 to 3.5 ng/mL.[33][91]
A study that investigated the pharmacokinetics of topical progesterone using either a hydrophilic-, lipophilic-, or emulsion-type base found that in all three cases the time to peak concentrations was around 4 hours.[10] The venous blood levels observed were very low with all three bases.[10]
The site of application of transdermal progesterone may significantly influence absorption.[92] A study observed a significant increase in serum levels of progesterone shortly after administration when it was applied as a topical ointment to the breasts but not when it was applied to other areas like the thigh or abdomen.[92]
Although very low levels of progesterone have been observed in venous blood with topical progesterone, very high and in fact greatly supraphysiological levels of progesterone have unexpectedly been found in saliva and capillary blood.[91][92][107] In one study, the levels of progesterone in saliva and capillary blood were 10- and 100-fold greater than levels in venous blood, respectively.[91][92][107] Levels of salivary progesterone that have been observed in clinical studies with transdermal progesterone have ranged from 2.9 to 2,840 ng/mL.[33] High salivary and capillary blood levels of progesterone with topical progesterone suggest that despite low circulating levels of progesterone, systemic distribution of progesterone and considerable exposure of some tissues to the hormone may be occurring somehow.[91][92][107] However, the few clinical studies that have assessed the effects of topical progesterone on the endometrium have had mixed findings, and further research is needed to determine whether topical progesterone can confer adequate endometrial protection as a component of menopausal hormone therapy.[91][92]
Topical progesterone is usually supplied in the form of creams and water-based gels, and the studies in which very low levels of progesterone in circulation were observed with topical progesterone used these formulations.[91][92] A study of 100 mg/day topical progesterone in the form of an alcohol-based gel found relatively high concentrations of progesterone in circulation that corresponded to luteal-phase levels.[91][92] The peak levels of progesterone were 8 ng/mL and were theoretically sufficient to confer endometrial protection.[91][92] Though based on limited data, these findings suggest that alcohol-based progesterone gels may yield relatively high levels of circulating progesterone.[91][92] One possible explanation for the difference is that progesterone creams are more lipophilic and may have a preference for uptake into the fatty layer under the skin whereas alcohol-based gels are more water-soluble and may rapidly distribute into the microcirculation of the skin and then into the general circulation.[92]
High levels in saliva and capillary blood
On the basis of the very low levels of progesterone observed in venous blood with topical progesterone, some researchers have concluded that topical progesterone is not well-absorbed and will not allow for adequate endometrial protection.[107][92] However, in spite of very low levels of progesterone in circulation with topical progesterone, studies that have measured levels of progesterone in saliva and/or capillary blood have found that they are dramatically elevated and in fact greatly supraphysiological.[91][92][107] In one study that used an oil-based cream or water-based gel, salivary and fingertip capillary blood levels of progesterone were found to be approximately 10-fold and 100-fold greater than venous blood levels, respectively.[91][107] The exact levels of progesterone were 4 to 12 ng/mL in saliva and 62 to 96 ng/mL in capillary blood; the reference ranges of progesterone in saliva and capillary blood from a cited laboratory were 0.75 to 2.5 ng/mL and 3.3 to 22.5 ng/mL for premenopausal women in the luteal phase and 0.12 to 1.0 ng/mL and 0.1 to 0.8 ng/mL in postmenopausal women, respectively.[91][107] As such, these data confirm distribution of progesterone to at least certain tissues with topical progesterone in spite of very low levels of progesterone in circulation and indicate that progesterone levels in venous blood cannot necessarily be used as an index of tissue exposure to progesterone with this route of administration.[91][92] These findings provide a possible explanation for how some studies found antiproliferative and atrophic changes in the endometrium with topical progesterone.[107][92] However, elevated levels of progesterone in the endometrium with topical progesterone have yet to be demonstrated.[92]
Concern has been raised regarding topical progesterone in that the effects of such supraphysiological levels of progesterone in tissues are unknown and hence the potential for adverse effects has not been ruled out.[91] Salivary monitoring of progesterone levels in women using topical progesterone and adjustment of dosage as necessary has been suggested as a possible means to help prevent potential adverse effects.[91]
The mechanism by which topical progesterone in cream and water-based gel produces very high salivary and capillary blood levels in spite of low circulating levels is not well-understood.[91] However, at least two hypotheses have been proposed.[92][108] Steroid hormones including progesterone have been found to be transported by red blood cells in addition to serum carrier proteins like albumin, sex hormone-binding globulin, and corticosteroid-binding globulin, and as much as 15 to 35% of total steroid hormone content in whole blood may be confined to red blood cells.[92] According to the hypothesis, very high local concentrations of progesterone occur in skin capillaries after topical application and are taken up by red blood cells.[92] The transit time of red blood cells from capillaries and the release of steroid hormones from red blood cells are both very rapid, so it is suggested that progesterone is delivered through circulation to tissues via red blood cells without having time to equilibrate with systemic blood.[92] This could potentially explain the low levels of progesterone in venous blood in spite of very high levels in capillary blood and saliva.[92] However, one study assessed progesterone levels in red blood cells with topical progesterone and found that they were significantly increased but still very low.[92] Nonetheless, according to other authors, "[a]lthough the investigators of that study concluded that the progesterone levels in red blood cells were too low to be important in the delivery of progesterone to target tissues, it should be realized that even small amounts of progesterone taken up by red blood cells might be important because the transit time of red blood cells from capillaries is very rapid. [...] However, the role of red blood cells in steroid hormone transport has not been studied thoroughly, and such studies are warranted."[92]
An in vitro study using porcine skin and several formulations of topical progesterone found that only minute quantities of progesterone penetrated through the skin but that there was significant partitioning of progesterone in the skin tissues.[108] According to the researchers, the results suggested that lymphatic circulation in the skin might account for systemic distribution of topical progesterone.[108]
Metabolism and elimination
5α-Reductase is an important and major enzyme involved in the metabolism of progesterone, and skin is known to express high levels of this enzyme.[92] As such, it has been suggested that rapid metabolism of progesterone by 5α-reductase could account for the low levels of circulating progesterone produced by topical application with creams or water-based gels.[92] However, doubt has been cast on this hypothesis for several reasons.[92] For instance, topical progesterone in an alcohol-based gel has been found to produce high levels of circulating progesterone.[92] In addition, a study assessed urinary levels of pregnanediol glucuronide, a 5α-reduced metabolite of progesterone and the major metabolite of progesterone in urine, and found that although urinary levels of the metabolite increased after treatment with topical progesterone (similarly to circulating levels of progesterone), the levels nonetheless remained in the range of the follicular phase and hence remained very low.[92] Moreover, a case report found that the 5α-reductase inhibitor finasteride did not increase the circulating progesterone levels or urinary pregnanediol glucuronide levels produced by topical progesterone.[92] Finally, 5α-reductase is also a major enzyme involved in the metabolism of testosterone, yet topical testosterone in the form of creams, gels, and patches is approved for use as a pharmaceutical drug and is well-established as effective in raising circulating testosterone levels.[109]
A study that investigated the pharmacokinetics of topical progesterone using either a hydrophilic-, lipophilic-, or emulsion-type base found that in all three cases the elimination half-life was in the range of 30 to 40 hours.[10]
Systemic clinical effectiveness
At least seven studies have assessed topical progesterone.[91][92] In these studies, different formulations of topical progesterone including creams and water-based gels (brand names Pro-Gest, Progestelle, and Pro-Femme, as well as compounded) were used, with different sample sizes (n = 6 to n = 40), at different dosages (15 to 80 mg per day), and for different durations of treatment (1.4 to 24 weeks).[91][92] Venous blood progesterone levels were assessed and reported in five of the studies and in all cases were low and found not to exceed 3.5 ng/mL.[91][92] It is generally accepted that progesterone levels of 5 ng/mL are necessary to inhibit mitosis and induce secretory changes in the endometrium,[91] although some researchers have been disputed this contention.[92] Effects on the endometrium of topical progesterone were assessed in three of the studies via endometrial biopsy and the results were mixed.[91][92] In one study, there was no effect; in another, antiproliferative effects were observed; and in the last study, an atrophic state was observed but only in 28 of 40 (70%) of the women.[91][92] Circulating progesterone levels were reported as less than 3.5 ng/mL in the first study, low and widely variable in the second study, and were not given in the third study.[91][92] Moreover, the duration of the study in which no effect was observed was short at only 2 weeks, and a longer treatment period of 4 to 6 weeks is necessary to produce endometrial changes.[91][92] It has also been suggested that the dosage of estrogen used may have been insufficient to allow for proper priming of the endometrium for progesterone to act.[92] Taken together, further studies are required to adequately establish a protective effect of topical progesterone on the endometrium.[91]
Local application to the breasts
Topical application of progesterone with the intention of systemic therapy should not be equated with local treatment.[33] The site of application of topical progesterone has been found to significantly influence its absorption.[92] When topical progesterone is applied to the breasts, high concentrations within breast tissue have been observed.[93] In one study, a 3- to 5-fold increase in local progesterone levels in the breast was observed with 50 mg topical progesterone in an alcohol/water-based gel applied to each breast in premenopausal women.[93][100][110] In another study, a 70- to 110-fold increase in local concentrations of progesterone in the breasts was measured with application of a hydroalcoholic gel to the breasts in premenopausal women.[111][112] A study observed a significant increase in circulating levels of progesterone when it was applied as a topical ointment to the breasts but not when it was applied to other areas like the thigh or abdomen.[92] However, two other studies observed no apparent increase in circulating levels of progesterone with topical application of progesterone to the breasts.[111][100] On the basis of its transdermal bioavailability when applied to the breasts of 10%, a 50 mg dose of progesterone applied transdermally may result in a local concentration of progesterone in the breasts of 5 mg.[93][110]
While topical progesterone is not approved for use in menopausal hormone therapy or as a systemic medication, it is registered in some countries under the brand name Progestogel as a 1% gel (10 mg/g) for direct local application to the breasts to treat premenstrual breast pain.[94][33][102] The medication has been found in clinical studies to inhibit estrogen-induced proliferation of breast epithelial cells, to be highly effective in the treatment of benign breast disease, to significantly decrease breast nodularity, and to almost completely alleviate breast pain and tenderness in women with the condition.[33][93][100][102] Conversely, topical progesterone has been found to be almost completely ineffective in fibrocystic breast disease, breast cysts, and breast fibroadenomas, whereas oral progestins were found to be significantly effective.[93] The effectiveness of progesterone and other progestogens in the treatment of breast disorders may be due to their functional antiestrogenic effects in the breasts.[93][100]
Vaginal administration
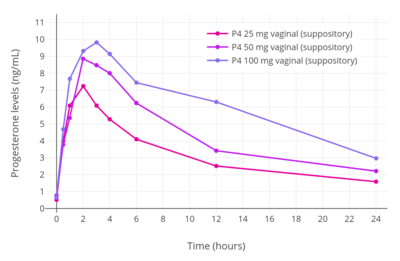
Progesterone is available for vaginal administration in the form of capsules (Utrogestan), gels (Crinone, Prochieve), suppositories (Cyclogest), inserts/tablets (Endometrin, Lutinus), and rings (Fertiring, Progering).[114][115][116]
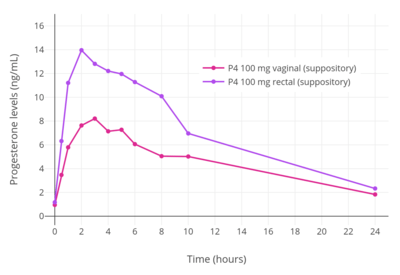
The bioavailability of vaginal micronized progesterone is about 4 to 8%.[2][3][4] Vaginal absorption of progesterone is lower in postmenopausal women with vaginal atrophy.[113] The bioavailability of vaginal progesterone is about 40-fold greater than that of oral progesterone.[117][1] Following administration of a single 25, 50, or 100 mg vaginal progesterone suppository in women, maximal circulating levels of progesterone occurred within 2 to 3 hours and were 7.27 ± 2.8 ng/mL, 8.84 ± 3.14 ng/mL, and 9.82 ± 9.8 ng/mL, respectively.[113] After peak levels, progesterone levels decreased gradually, with an elimination half-life of 6 to 12 hours.[113] Progesterone levels were less than 3 ng/mL for all three doses after 24 hours.[113] The researchers concluded that the 25 and 50 mg doses would be appropriate for twice daily administration while the 100 mg dose would be appropriate for administration three times a day.[113]
There is a uterine first-pass effect with vaginal progesterone, such that progesterone levels are far greater in the uterus than in the circulation.[33] Full secretory transformation of the endometrium was produced by vaginal progesterone administration that resulted in circulating progesterone levels of 1 to 3 ng/mL, whereas other parenteral routes (intramuscular and intranasal) were less effective in comparison.[113] The difference can be attributed to the endometrial first-pass effect with vaginal progesterone.[113]
Rectal administration
Progesterone can be taken by rectal administration.[118][17][25] A suppository sold under the brand name Cyclogest is indicated for rectal use in addition to the vaginal route.[35][119][120] Daily rectal administration of progesterone is inconvenient and poorly accepted for long-term therapy.[32][118] Nonetheless, rectal progesterone can be a useful alternative to the vaginal route in the context of vaginal infection, cystitis, recent childbirth, or when barrier contraception methods are used.[118]
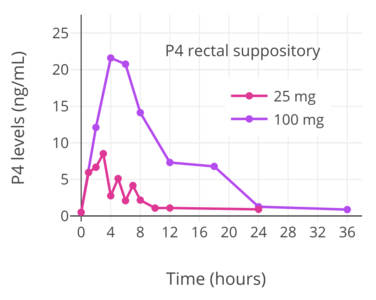
A number of studies have assessed progesterone by the rectal route.[121][122][123][124][125][89] Levels of progesterone following rectal administration have been found to be 6.4 ng/mL after a single 25 mg suppository, 22.5 ng/mL after a single 100 mg suppository, and 20.0 ng/mL after a single 200 mg suppository.[118][124] The absorption of the rectal route is variable, with a wide range of maximal concentrations of 15 to 52 ng/mL progesterone after a single rectal dose of 100 mg progesterone.[17][123] Levels of progesterone peak after 6 to 8 hours and then gradually decrease.[17][118] Progesterone levels have been found to be similar and non-significantly different after administration of rectal and vaginal suppositories in several studies.[118]
Progesterone is delivered directly into the circulation when it is absorbed by the lower portion of the rectum and transported by the inferior and middle rectal veins.[17] Conversely, if it is absorbed by the upper portion of the rectum, progesterone is subject to hepatic first-pass metabolism due to entry into the hepatic portal system via the superior rectal vein.[17] As such, although rectal administration is a parenteral route, it may still be subject to some first-pass metabolism similarly to oral progesterone.[17]
Intramuscular injection
Oil solution
When used by intramuscular injection, progesterone bypasses first-pass metabolism in the intestines and liver and achieves very high circulating progesterone levels.[16][33] Levels of progesterone with 100 mg/day intramuscular progesterone were substantially higher than with 800 mg/day vaginal progesterone (about 70 ng/mL and 12 ng/mL, respectively), although local progesterone levels in the uterus were 10 times higher with the vaginal route due to a uterine first-pass effect (around 1.5 ng/mL and almost 12 ng/mL, respectively).[33] The duration of progesterone is extended by the intramuscular route due to a depot effect in which it is stored locally in adipose tissue, and can be administered once every 1 to 3 days.[17] The half-life of intramuscular progesterone is significantly longer when it is injected into the gluteal muscles of the buttocks rather than the deltoid muscle of the upper arm.[17] Intramuscular progesterone has traditionally been the most popular form of progesterone used for luteal support in assisted reproduction in the United States, although vaginal progesterone is also used and effective.[33][17]
With intramuscular injection of 10 mg progesterone in vegetable oil, maximum plasma concentrations (Cmax) are reached at approximately 8 hours after administration, and serum levels remain above baseline for about 24 hours.[22] Doses of 10, 25, and 50 mg via intramuscular injection have been found to result in average maximal concentrations of 7, 28, and 50 ng/mL, respectively.[22] With intramuscular injection, a dose of 25 mg results in normal luteal phase serum levels of progesterone within 8 hours, and a 100 mg dose produces mid-pregnancy levels of 40 to 80 ng/mL at peak.[25] At these doses, levels of progesterone remain elevated above baseline for at least 48 hours (6 ng/mL at this point for 100 mg),[25] with an elimination half-life of about 22 hours.[12]
Due to the high concentrations achieved, progesterone by intramuscular injection at the usual clinical dose range is able to suppress gonadotropin secretion from the pituitary gland, demonstrating antigonadotropic efficacy (and therefore suppression of gonadal sex steroid production).[22]
Intramuscular progesterone often causes pain when injected.[17] It irritates tissues and is associated with injection site reactions such as changes in skin color, pain, redness, transient indurations (due to inflammation), ecchymosis (bruising/discoloration), and others.[126][17] Rarely, sterile abscesses can occur.[17]
| Progestogen | Form | Major brand names | Class | TFD (14 days) | POIC-D (2–3 months) | CIC-D (month) | Duration | |
|---|---|---|---|---|---|---|---|---|
| Algestone acetophenide | Oil solution | Perlutal, Topasel, Yectames | Pregnane | ? | – | 75–150 mg | 100 mg ≈ 14–32 days | |
| Cyproterone acetate | Oil solution | Androcur Depot | Pregnane | ? | – | – | 300 mg ≈ 20 days | |
| Dydrogesteronea | Aqueous suspension | – | Retropregnane | ? | – | – | 100 mg ≈ 16–38 days | |
| Gestonorone caproate | Oil solution | Depostat, Primostat | Norpregnane | 50 mg | – | – | 25–50 mg ≈ 8–13 days | |
| Hydroxyprogesterone acetatea | Aqueous suspension | – | Pregnane | 350 mg | – | – | 150–350 mg ≈ 9–16 days | |
| Hydroxyprogesterone caproate | Oil solution | Delalutin, Proluton, Makena | Pregnane | 250–500 mgb | – | 250–500 mg | 65–500 mg ≈ 5–21 days | |
| Levonorgestrel butanoatea | Aqueous suspension | – | Gonane | ? | – | – | 5–50 mg ≈ 3–6 months | |
| Lynestrenol phenylpropionatea | Oil solution | – | Estrane | ? | – | – | 50–100 mg ≈ 14–30 days | |
| Medroxyprogesterone acetate | Aqueous suspension | Depo-Provera | Pregnane | 50–100 mg | 150 mg | 25 mg | 50–150 mg ≈ 14–50+ days | |
| Megestrol acetate | Aqueous suspension | Mego-E | Pregnane | ? | – | 25 mg | 25 mg ≈ >14 daysc | |
| Norethisterone enanthate | Oil solution | Noristerat, Mesigyna | Estrane | 100–200 mg | 200 mg | 50 mg | 50–200 mg ≈ 11–52 days | |
| Oxogestone phenylpropionatea | Oil solution | – | Norpregnane | ? | – | – | 100 mg ≈ 19–20 days | |
| Progesterone | Oil solution | Progestaject, Gestone, Strone | Pregnane | 200 mgb | – | – | 25–350 mg ≈ 2–6 days | |
| Aqueous suspension | Agolutin Depot | Pregnane | 50–200 mg | – | – | 50–300 mg ≈ 7–14 days | ||
| Note: All by intramuscular or subcutaneous injection. All are synthetic except for P4, which is bioidentical. P4 production during the luteal phase is ~25 (15–50) mg/day. The OID of OHPC is 250 to 500 mg/month. Footnotes: a = Never marketed by this route. b = In divided doses (2 × 125 or 250 mg for OHPC, 10 × 20 mg for P4). c = Half-life is ~14 days. Sources: Main: See template. | ||||||||
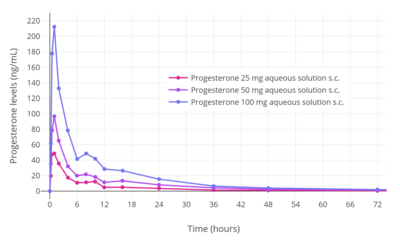
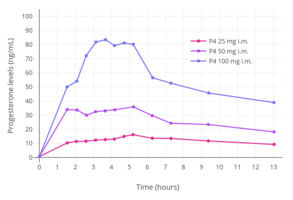 Progesterone levels with a single intramuscular injection of 25, 50, or 100 mg progesterone (P4) in oil solution in postmenopausal women.[63]
Progesterone levels with a single intramuscular injection of 25, 50, or 100 mg progesterone (P4) in oil solution in postmenopausal women.[63]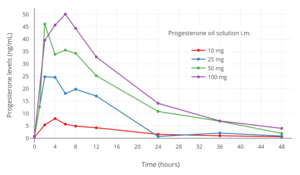 Progesterone levels with a single intramuscular injection of 10, 25, 50, or 100 mg progesterone in oil solution in women.[125]
Progesterone levels with a single intramuscular injection of 10, 25, 50, or 100 mg progesterone in oil solution in women.[125]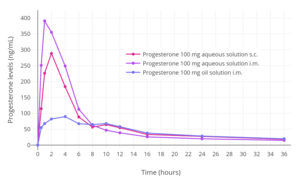 Progesterone levels following a single intramuscular or subcutaneous injection of 100 mg progesterone in an aqueous solution (Prolutex) or oil solution (Prontogest) in postmenopausal women.[12]
Progesterone levels following a single intramuscular or subcutaneous injection of 100 mg progesterone in an aqueous solution (Prolutex) or oil solution (Prontogest) in postmenopausal women.[12]
Aqueous suspension
Progesterone has been found to have a considerably longer duration of action by intramuscular injection when administered in the form of a microcrystalline aqueous suspension (crystal sizes of 0.02–0.1 mm) than as an oil solution.[127][128][129][130][131] Whereas a single intramuscular injection of 25 to 350 mg progesterone in oil solution has a duration of 2 to 6 days in terms of clinical biological effect in the uterus in women, a single intramuscular injection of 50 to 300 mg microcrystalline progesterone in aqueous suspension has a duration of 7 to 14 days.[127][128][129] As a result, intramuscular progesterone in oil solution is given once every 1 to 3 days at typical clinical doses,[17] whereas intramuscular microcrystalline progesterone in aqueous suspension can be given once weekly or at even longer intervals.[127][129][132] The duration of microcrystalline aqueous suspensions is dependent both on drug concentration and on crystal size.[133][134][135][136]
Formulations of microcrystalline progesterone in aqueous suspension for long-lasting depot use via intramuscular injection were on the market in the 1950s under a variety of brand names including Flavolutan, Luteosan, Lutocyclin M, and Lutren.[137] Another preparation is Agolutin Depot, which was introduced by 1960 and appears to remain marketed in the Czech Republic and Slovakia today.[138][139][140][130][141] Sistocyclin was the brand name of a product containing 10 mg microcrystalline estradiol benzoate and 200 mg microcrystalline progesterone in an aqueous suspension which was marketed in the 1950s.[142][143][144][145] The medication was reported to have a duration of action of 10 to 12 days in terms of the progestogen component, relative to a duration of only 2 days for amorphous estradiol benzoate and progesterone in oil solution.[146][147]
Intramuscular injections of microcrystalline progesterone in aqueous suspension is painful, often severely so.[148][149][150] As a result, they were discontinued in favor of other preparations, such as progesterone in oil solution.[148][149][150]
Medroxyprogesterone acetate (brand names Depo-Provera, Depo-SubQ Provera 104), a progestin and structural modification of progesterone with a methyl group at the C6α position and an acetoxy group at the C17α position, is formulated as a microcrystalline aqueous suspension for use by intramuscular or subcutaneous injection.[151][152] As with progesterone, the formulation of medroxyprogesterone acetate in this way dramatically extends its duration.[151][152] It has a duration of 16 to 50 days at a dose of 50 mg[127] and at least 3 months and to as long as 6 to 9 months at a dose of 150 mg.[151][152]
Microspheres
An aqueous suspension formulation of progesterone contained in microspheres for use by intramuscular injection is marketed under the brand name ProSphere in Mexico.[126][153][154] It is much longer-lasting than regular intramuscular progesterone and is administered once weekly or once monthly, depending on the indication.[126] A combination of both estradiol and progesterone encapsulated within microspheres as an aqueous suspension for use by intramuscular injection has been marketed under the brand name Juvenum in Mexico as well.[155][156][157] Studies of this formulation have been published.[158][159]
Estradiol and progesterone encapsulated in microspheres has been studied for use as a once-a-month combined injectable contraceptive but has not been further developed nor introduced for medical use.[160][161][162][163][164][165]
Subcutaneous injection
Progesterone can be administered by subcutaneous injection, with Prolutex, an aqueous solution of progesterone marketed in Europe, being intended for once-daily administration by this route.[12][166][167] This formulation is rapidly absorbed and has been found to result in higher peak levels of progesterone relative to progesterone in oil solution by intramuscular injection.[167] In addition, subcutaneous injection of progesterone is considered to be easier, safer due less risk of injection site reactions, and less painful compared to intramuscular injection of progesterone.[167] The elimination half-life of this formulation is 13 to 18 hours,[12] compared to 20 to 28 hours for intramuscular injection of progesterone in oil solution.[11][9][12]
Subcutaneous implantation
Progesterone was previously marketed in the 1950s and 1960s in the form of 50 and 100 mg subcutaneous pellet implants under the brand names Flavolutan, Luteosid, Lutocyclin, and Proluton.[137][168] However, in contrast to estradiol and testosterone implants, which remain available as pharmaceutical products today,[169] progesterone implant products have been discontinued and appear to no longer be available pharmaceutically.[170] Progesterone implants may be available from some compounding pharmacies however, although such products are not regulated for quality or effectiveness.[171][172][173]
Early studies of progesterone implants in humans were conducted in the 1930s to 1950s.[174][175][176][177][178][179][180][181] Subcutaneous implants of progesterone were found to be poorly tolerated, with sterile abscesses and extrusion occurring in 15 to 20% of implantations.[182] Progesterone implants were also studied as a form of long-lasting hormonal birth control in women in the 1980s, but ultimately were never marketed.[183][184][185][186] Implantation of six pellets containing 100 mg progesterone each (600 mg total) has been found to result in relatively low mean progesterone levels of about 3 ng/mL, with progesterone levels sustained for five months.[184][185][186] Subcutaneous implantation of progesterone has been studied in animals as well.[187]
Although progesterone implants are not available as pharmaceutical preparations, subcutaneous implants of progestins, such as etonogestrel (Implanon/Nexplanon) and levonorgestrel (Jadelle/Norplant), are available as pharmaceutical products.[188][189] They are used as forms of long-lasting hormonal birth control.[188][189]
Intrauterine administration
A one-year progesterone intrauterine device (IUD) for hormonal birth control was previously available in the United States and a few other countries under the brand name Progestasert.[190][191] It was marketed between 1976 and 2001.[190] The IUD was never widely used due to a relatively high contraceptive failure rate of 2.9% and the requirement of annual replacement.[190] It contained 38 mg progesterone and released 65 μg progesterone into the uterus per day (totaling up to about 35 mg after one year).[190][191] For comparison, a woman's body produces on average about 25 mg progesterone per day during the luteal phase.[17][83] While effective as a form of contraception and for decreasing menstrual bleeding and discomfort, long-lived IUDs can fundamentally only deliver small amounts of progesterone per day, and hence intrauterine progesterone cannot achieve adequate circulating progesterone levels and is unsuitable as a form of systemic therapy.[83] Aside from progesterone, IUDs of progestins, such as levonorgestrel (Mirena/Levosert/Skyla), are available as well.[192]
Intravenous injection
Progesterone has a very short elimination half-life of about 3 to 90 minutes when given by intravenous injection.[13]
General
Absorption
The absorption of progesterone varies depending on the route of administration.[16]
Distribution
Progesterone crosses the blood–brain barrier.[193] In terms of plasma protein binding, progesterone is 98 to 99% protein-bound in the circulation.[5][6] It is bound 80% to albumin, 18% to corticosteroid-binding globulin, and less than 1% to sex hormone-binding globulin, with the remaining fraction of 1 to 2% circulating freely or unbound.[5][6]
Metabolism
With oral administration, progesterone is rapidly metabolized in the gastrointestinal tract and liver.[94] As many as 30 different metabolites have been found to be formed from progesterone with oral ingestion.[94] Regardless of the route of administration, 5α-reductase is the major enzyme involved in the metabolism of progesterone and is responsible for approximately 60 to 65% of its metabolism.[60] 5β-Reductase is also a major enzyme in the metabolism of progesterone.[60] 5α-Reduction of progesterone occurs predominantly in the intestines (specifically the duodenum), whereas 5β-reduction occurs almost exclusively in the liver.[60] The metabolites of progesterone produced by 5α-reductase and 5β-reductase (after further transformation by 3α-hydroxysteroid dehydrogenase) are allopregnanolone and pregnanolone, respectively.[94] With oral administration of progesterone, they occur in circulation at very high and in fact supraphysiological concentrations that are well in excess of those of progesterone itself (peak concentrations of 30 ng/mL for allopregnanolone and 60 ng/mL for pregnanolone versus 12 ng/mL for progesterone at 4 hours after a single 200-mg oral dose of progesterone).[94] In one study, a single 200-mg oral dose of progesterone resulted in peak levels of 20α-dihydroprogesterone of around 1 ng/mL after 2 hours.[194]
The percentage constitutions of progesterone and its metabolites as reflected in serum levels have been determined for a single 100 mg dose of oral or vaginal progesterone.[50] With oral administration, progesterone accounts for less than 20% of the dose in circulation while 5α- and 5β-reduced products like allopregnanolone and pregnanolone account for around 80%.[50] With vaginal administration, progesterone accounts for around 50% of the dose and 5α- and 5β-reduced metabolites for around 40%.[50]
A small amount of progesterone is converted by 21-hydroxylase into 11-deoxycorticosterone.[195][60] Increases in levels of 11-deoxycorticosterone are markedly higher when progesterone is given orally as opposed to via parenteral routes like vaginal or intramuscular injection.[60] The conversion of progesterone into 11-deoxycorticosterone occurs in the intestines (specifically the duodenum) and in the kidneys.[195][60] 21-Hydroxylase appears to be absent in the liver, so conversion of progesterone into 11-deoxycorticosterone is thought not to occur in this part of the body.[60]
Endogenous progesterone is metabolized approximately 50% into 5α-dihydroprogesterone in the corpus luteum, 35% into 3β-dihydroprogesterone in the liver, and 10% into 20α-dihydroprogesterone.[46]
Metabolites of progesterone with one or more available hydroxyl groups are conjugated via glucuronidation and/or sulfation and excreted.[196][35]
The biological half-life of progesterone in the circulation is very short; with intravenous injection, its half-life has ranged widely from 3 to 90 minutes in various studies.[13] The metabolic clearance rate of progesterone ranges between 2,100 and 2,800 L/day, and is constant across the menstrual cycle.[13][150]
Metabolism of progesterone in humans[13]
|
Elimination
Progesterone is eliminated in bile and urine.[14][15]
See also
References
- Levine H, Watson N (March 2000). "Comparison of the pharmacokinetics of Crinone 8% administered vaginally versus Prometrium administered orally in postmenopausal women(3)". Fertil. Steril. 73 (3): 516–21. doi:10.1016/S0015-0282(99)00553-1. PMID 10689005.
- Griesinger G, Tournaye H, Macklon N, Petraglia F, Arck P, Blockeel C, van Amsterdam P, Pexman-Fieth C, Fauser BC (February 2019). "Dydrogesterone: pharmacological profile and mechanism of action as luteal phase support in assisted reproduction". Reprod. Biomed. Online. 38 (2): 249–259. doi:10.1016/j.rbmo.2018.11.017. PMID 30595525.
- Pandya MR, Gopeenathan P, Gopinath PM, Das SK, Sauhta M, Shinde V (2016). "Evaluating the clinical efficacy and safety of progestogens in the management of threatened and recurrent miscarriage in early pregnancy-A review of the literature". Indian Journal of Obstetrics and Gynecology Research. 3 (2): 157. doi:10.5958/2394-2754.2016.00043.6. ISSN 2394-2746.
- Paulson RJ, Collins MG, Yankov VI (November 2014). "Progesterone pharmacokinetics and pharmacodynamics with 3 dosages and 2 regimens of an effervescent micronized progesterone vaginal insert". J. Clin. Endocrinol. Metab. 99 (11): 4241–9. doi:10.1210/jc.2013-3937. PMID 24606090.
- Fritz MA, Speroff L (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 44–. ISBN 978-1-4511-4847-3.
- Marshall WJ, Marshall WJ, Bangert SK (2008). Clinical Chemistry. Elsevier Health Sciences. pp. 192–. ISBN 0-7234-3455-7.
- Pickar JH, Bon C, Amadio JM, Mirkin S, Bernick B (December 2015). "Pharmacokinetics of the first combination 17β-estradiol/progesterone capsule in clinical development for menopausal hormone therapy". Menopause. 22 (12): 1308–16. doi:10.1097/GME.0000000000000467. PMC 4666011. PMID 25944519.
- Хомяк, Н. В., Мамчур, В. И., & Хомяк, Е. В. (2014). Клинико-фармакологические особенности современных лекарственных форм микронизированного прогестерона, применяющихся во время беременности. Здоровье, (4), 90. https://web.archive.org/web/20180808140010/http://health-ua.com/wp-content/uploads/2015/09/MAZG2-2015_28-35.pdf
- http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020701s026lbl.pdf
- Mircioiu C, Perju A, Griu E, Calin G, Neagu A, Enachescu D, Miron DS (1998). "Pharmacokinetics of progesterone in postmenopausal women: 2. Pharmacokinetics following percutaneous administration". Eur J Drug Metab Pharmacokinet. 23 (3): 397–402. doi:10.1007/bf03192300. PMID 9842983.
- Simon JA, Robinson DE, Andrews MC, Hildebrand JR, Rocci ML, Blake RE, Hodgen GD (1993). "The absorption of oral micronized progesterone: the effect of food, dose proportionality, and comparison with intramuscular progesterone". Fertil. Steril. 60 (1): 26–33. doi:10.1016/S0015-0282(16)56031-2. PMID 8513955.
- Cometti B (November 2015). "Pharmaceutical and clinical development of a novel progesterone formulation". Acta Obstetricia et Gynecologica Scandinavica. 94 Suppl 161: 28–37. doi:10.1111/aogs.12765. PMID 26342177.
- Aufrère MB, Benson H (June 1976). "Progesterone: an overview and recent advances". J Pharm Sci. 65 (6): 783–800. doi:10.1002/jps.2600650602. PMID 945344.
- http://www.accessdata.fda.gov/drugsatfda_docs/label/1998/20843lbl.pdf
- http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/017362s104lbl.pdf
- Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Unfer V, di Renzo GC, Gerli S, Casini ML (2006). "The Use of Progesterone in Clinical Practice: Evaluation of its Efficacy in Diverse Indications Using Different Routes of Administration". Current Drug Therapy. 1 (2): 211–219. doi:10.2174/157488506776930923.
- Whitaker A, Gilliam M (2014). Contraception for Adolescent and Young Adult Women. Springer. p. 98. ISBN 9781461465799.
- Chaudhuri SK (2007). Practice Of Fertility Control: A Comprehensive Manual (7th ed.). Elsevier India. p. 153. ISBN 978-81-312-1150-2.
- Zutshi V, Rathore AM, Sharma K (2005). Hormones in Obstetrics and Gynaecology. Jaypee Brothers, Medical Publishers. pp. 74–75. ISBN 978-81-8061-427-9.
It has been observed that micronized progesterone has no suppressive effects on high-density lipoprotein-cholesterol (HDL-C). Jensen et al have proved that oral micronized progesterone has no adverse effect on serum lipids. These preparations have the same antiestrogenic and antimineralocorticoid effect but no androgenic action. It does not affect aldosterone synthesis, blood pressure, carbohydrate metabolism or mood changes. No side effects have been reported as far as lipid profile, coagulation factors and blood pressure are concerned.
- Lark S (1999). Making the Estrogen Decision. McGraw-Hill Professional. p. 22. ISBN 9780879836962.
- Progesterone - Drugs.com, retrieved 2015-08-23
- Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P, Stricker R (2006). "Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer". Clin. Chem. Lab. Med. 44 (7): 883–7. doi:10.1515/CCLM.2006.160. PMID 16776638.
- Josimovich J (11 November 2013). Gynecologic Endocrinology. Springer Science & Business Media. pp. 9, 25–29, 139. ISBN 978-1-4613-2157-6.
- van Keep PA, Utian WH (6 December 2012). The Premenstrual Syndrome: Proceedings of a workshop held during the Sixth International Congress of Psychosomatic Obstetrics and Gynecology, Berlin, September 1980. Springer Science & Business Media. pp. 51–52. ISBN 978-94-011-6255-5.
- Strauss JF, Barbieri RL (2009). Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Elsevier Health Sciences. pp. 807–. ISBN 1-4160-4907-X.
- Blackburn S (14 April 2014). Maternal, Fetal, & Neonatal Physiology. Elsevier Health Sciences. pp. 92–. ISBN 978-0-323-29296-2.
- Kuhl H, Schneider HP (August 2013). "Progesterone – promoter or inhibitor of breast cancer". Climacteric. 16 Suppl 1: 54–68. doi:10.3109/13697137.2013.768806. PMID 23336704.
- Kuhl H (2011). "Pharmacology of Progestogens" (PDF). Journal für Reproduktionsmedizin und Endokrinologie-Journal of Reproductive Medicine and Endocrinology. 8 (1): 157–177.
- Davey DA (March 2018). "Menopausal hormone therapy: a better and safer future". Climacteric. 21: 1–8. doi:10.1080/13697137.2018.1439915. PMID 29526116.
- Fotherby K (August 1996). "Bioavailability of orally administered sex steroids used in oral contraception and hormone replacement therapy". Contraception. 54 (2): 59–69. doi:10.1016/0010-7824(96)00136-9. PMID 8842581.
Progesterone is rapidly metabolized which leads to a low bioavailability and the necessity for high doses (200 mg); its pharmacological actions, except upon reproductive processes, are weak. [...] For many years after its isolation and synthesis, progesterone could only be used therapeutically by intramuscular injection of large doses since it was quickly recognized that, after oral administration, progesterone was poorly absorbed and rapidly metabolized in the gastrointestinal tract, liver and most tissues, and then eliminated. However, like estradiol, the absorption of progesterone can be improved by micronization.16 This led to the development of formulations of progesterone, mainly of particle size <10 μg, dissolved in oil in a gelatin capsule. [...] five formulations differing in particle size and vehicle were compared; all led to significant absorption of progesterone and with similar plasma progesterone concentrations 6 hours after administration, but only the micronized or oil vehicle preparations improved absorption (probably by increasing lymphatic absorption and thus avoiding some of the first-pass effect). [...] Plasma progesterone concentrations may be higher when the formulation is administered with food but, even under the best conditions, bioavailability appears to be very low since the AUC after intramuscular injection of progesterone is at least ten times higher than after oral intake.21
- de Lignières B (1999). "Oral micronized progesterone". Clin Ther. 21 (1): 41–60, discussion 1–2. doi:10.1016/S0149-2918(00)88267-3. PMID 10090424.
- Ruan X, Mueck AO (November 2014). "Systemic progesterone therapy—oral, vaginal, injections and even transdermal?". Maturitas. 79 (3): 248–55. doi:10.1016/j.maturitas.2014.07.009. PMID 25113944.
- Wecker L (31 May 2018). Brody's Human Pharmacology E-Book. Elsevier Health Sciences. pp. 419–. ISBN 978-0-323-59662-6.
- Anita MV, Jain S, Goel N (31 July 2018). Use of Progestogens in Clinical Practice of Obstetrics and Gynecology. JP Medical Ltd. pp. 4, 15–20. ISBN 978-93-5270-218-3.
Progesterone is also available in the forms of vaginal or rectal suppositories or pessaries (Cyclogest), [...]
- Hauss DJ (8 June 2007). Oral Lipid-Based Formulations: Enhancing the Bioavailability of Poorly Water-Soluble Drugs. CRC Press. pp. 17–. ISBN 978-1-4200-1726-7.
- Micozzi MS, Dog TL (19 August 2004). Women's Health in Complementary and Integrative Medicine E-Book: A Clinical Guide. Elsevier Health Sciences. pp. 69–. ISBN 0-7020-3598-X.
- Porter CJ, Pouton CW, Cuine JF, Charman WN (March 2008). "Enhancing intestinal drug solubilisation using lipid-based delivery systems". Adv. Drug Deliv. Rev. 60 (6): 673–91. doi:10.1016/j.addr.2007.10.014. PMID 18155801.
- Humberstone AJ, Charman WN (1997). "Lipid-based vehicles for the oral delivery of poorly water soluble drugs". Advanced Drug Delivery Reviews. 25 (1): 103–128. doi:10.1016/S0169-409X(96)00494-2. ISSN 0169-409X.
- Kincl FA, Ciaccio LA, Benagiano G (January 1978). "Increasing oral bioavailability of progesterone by formulation". J. Steroid Biochem. 9 (1): 83–4. doi:10.1016/0022-4731(78)90106-1. PMID 628211.
- Hargrove JT, Maxson WS, Wentz AC (October 1989). "Absorption of oral progesterone is influenced by vehicle and particle size". Am. J. Obstet. Gynecol. 161 (4): 948–51. doi:10.1016/0002-9378(89)90759-X. PMID 2801843.
- Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH (2008). "Classification and pharmacology of progestins" (PDF). Maturitas. 61 (1–2): 171–80. doi:10.1016/j.maturitas.2008.11.013. PMID 19434889.
- Hermann AC, Nafziger AN, Victory J, Kulawy R, Rocci ML, Bertino JS (2005). "Over-the-counter progesterone cream produces significant drug exposure compared to a food and drug administration-approved oral progesterone product". J Clin Pharmacol. 45 (6): 614–9. doi:10.1177/0091270005276621. PMID 15901742.
- Jameson JL, De Groot LJ (25 February 2015). Endocrinology: Adult and Pediatric E-Book. Elsevier Health Sciences. pp. 2660–. ISBN 978-0-323-32195-2.
- McAuley JW, Kroboth FJ, Kroboth PD (1996). "Oral administration of micronized progesterone: a review and more experience". Pharmacotherapy. 16 (3): 453–7. doi:10.1002/j.1875-9114.1996.tb02977.x. PMID 8726605.
- Anderson GD, Odegard PS (October 2004). "Pharmacokinetics of estrogen and progesterone in chronic kidney disease". Adv Chronic Kidney Dis. 11 (4): 357–60. doi:10.1053/j.ackd.2004.07.001. PMID 15492972.
- Integrative Medicine. Elsevier Health Sciences. 2012. p. 343. ISBN 1-4377-1793-4.
- Sauer MV (1 March 2013). Principles of Oocyte and Embryo Donation. Springer Science & Business Media. pp. 117–. ISBN 978-1-4471-2392-7.
- Alam V, Vega M, Rísquez F (2001). "Luteal phase support". Reprod. Biomed. Online. 3 (3): 250–262. doi:10.1016/S1472-6483(10)62044-5. PMID 12513863.
- de Ziegler D, Fanchin R (2000). "Progesterone and progestins: applications in gynecology". Steroids. 65 (10–11): 671–9. doi:10.1016/s0039-128x(00)00123-9. PMID 11108875.
[...] fairly low plasma levels of progesterone have been reported when the hormone is given orally, and proper assays are used. Circulating levels of progesterone and its metabolites, determined by sufficiently specific assays, after oral and vaginal administration of 100 mg of progesterone are illustrated in Fig. 4. As can be seen, when taken orally, progesterone accounts for less than 10%, whereas most of ingested progesterone is transformed to 5α-reduced metabolites. The metabolites of progesterone that bind to the GABAA receptor complex are responsible for drowsiness and other neurologic side effects. [See also Figure 4 for bar graphs of metabolites.]
- Frank R, Guterman HS (1954). "Comparison of progesterone preparations in secondary amenorrhea". Fertil. Steril. 5 (4): 374–81. doi:10.1016/S0015-0282(16)31687-9. PMID 13183192.
- Hohl A (30 March 2017). Testosterone: From Basic to Clinical Aspects. Springer. pp. 13–. ISBN 978-3-319-46086-4.
- Nieschlag E, Behre HM, Nieschlag S (26 July 2012). Testosterone: Action, Deficiency, Substitution. Cambridge University Press. pp. 313–. ISBN 978-1-107-01290-5.
- Bagchus WM, Hust R, Maris F, Schnabel PG, Houwing NS (March 2003). "Important effect of food on the bioavailability of oral testosterone undecanoate". Pharmacotherapy. 23 (3): 319–25. doi:10.1592/phco.23.3.319.32104. PMID 12627930.
- Schnabel PG, Bagchus W, Lass H, Thomsen T, Geurts TB (April 2007). "The effect of food composition on serum testosterone levels after oral administration of Andriol Testocaps". Clin. Endocrinol. (Oxf). 66 (4): 579–85. doi:10.1111/j.1365-2265.2007.02781.x. PMC 1859980. PMID 17371478.
- de Lignieres B, Dennerstein L, Backstrom T (April 1995). "Influence of route of administration on progesterone metabolism". Maturitas. 21 (3): 251–7. doi:10.1016/0378-5122(94)00882-8. PMID 7616875.
- Wang-Cheng R, Neuner JM, Barnabei VM (2007). Menopause. ACP Press. p. 97. ISBN 978-1-930513-83-9.
- Bergemann N, Ariecher-Rössler A (27 December 2005). Estrogen Effects in Psychiatric Disorders. Springer Science & Business Media. p. 179. ISBN 978-3-211-27063-9.
- Reddy DS (2010). "Neurosteroids: endogenous role in the human brain and therapeutic potentials". Progress in Brain Research. 186: 113–37. doi:10.1016/B978-0-444-53630-3.00008-7. PMC 3139029. PMID 21094889.
- Nahoul K, Dehennin L, Jondet M, Roger M (May 1993). "Profiles of plasma estrogens, progesterone and their metabolites after oral or vaginal administration of estradiol or progesterone". Maturitas. 16 (3): 185–202. doi:10.1016/0378-5122(93)90064-O. PMID 8515718.
- Warren MP, Shantha S (1999). "Uses of progesterone in clinical practice". Int J Fertil Womens Med. 44 (2): 96–103. PMID 10338267.
- Söderpalm AH, Lindsey S, Purdy RH, Hauger R, Wit de H (2004). "Administration of progesterone produces mild sedative-like effects in men and women". Psychoneuroendocrinology. 29 (3): 339–54. doi:10.1016/s0306-4530(03)00033-7. PMID 14644065.
- de Wit H, Schmitt L, Purdy R, Hauger R (2001). "Effects of acute progesterone administration in healthy postmenopausal women and normally-cycling women". Psychoneuroendocrinology. 26 (7): 697–710. doi:10.1016/s0306-4530(01)00024-5. PMID 11500251.
- van Broekhoven F, Bäckström T, Verkes RJ (2006). "Oral progesterone decreases saccadic eye velocity and increases sedation in women". Psychoneuroendocrinology. 31 (10): 1190–9. doi:10.1016/j.psyneuen.2006.08.007. PMID 17034954.
- Turkmen S, Backstrom T, Wahlstrom G, Andreen L, Johansson IM (2011). "Tolerance to allopregnanolone with focus on the GABA-A receptor". Br. J. Pharmacol. 162 (2): 311–27. doi:10.1111/j.1476-5381.2010.01059.x. PMC 3031054. PMID 20883478.
- Timby E, Balgård M, Nyberg S, Spigset O, Andersson A, Porankiewicz-Asplund J, Purdy RH, Zhu D, Bäckström T, Poromaa IS (2006). "Pharmacokinetic and behavioral effects of allopregnanolone in healthy women". Psychopharmacology. 186 (3): 414–24. doi:10.1007/s00213-005-0148-7. PMID 16177884.
- Pearlstein T (2016). "Treatment of Premenstrual Dysphoric Disorder: Therapeutic Challenges". Expert Rev Clin Pharmacol. 9: 1–4. doi:10.1586/17512433.2016.1142371. PMID 26766596.
A recent study with a 5α-reductase inhibitor dutasteride, that blocks the conversion of progesterone to ALLO, reported that dutasteride 2.5 mg daily decreased several premenstrual symptoms [7].
- Sripada RK, Marx CE, King AP, Rampton JC, Ho SS, Liberzon I (2013). "Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits". Biol. Psychiatry. 73 (11): 1045–53. doi:10.1016/j.biopsych.2012.12.008. PMC 3648625. PMID 23348009.
- Ducharme N, Banks WA, Morley JE, Robinson SM, Niehoff ML, Mattern C, Farr SA (2010). "Brain distribution and behavioral effects of progesterone and pregnenolone after intranasal or intravenous administration". Eur. J. Pharmacol. 641 (2–3): 128–34. doi:10.1016/j.ejphar.2010.05.033. PMC 3008321. PMID 20570588.
- Saudan C, Desmarchelier A, Sottas PE, Mangin P, Saugy M (2005). "Urinary marker of oral pregnenolone administration". Steroids. 70 (3): 179–83. doi:10.1016/j.steroids.2004.12.007. PMID 15763596.
- Piper T, Schlug C, Mareck U, Schänzer W (2011). "Investigations on changes in ¹³C/¹²C ratios of endogenous urinary steroids after pregnenolone administration". Drug Test Anal. 3 (5): 283–90. doi:10.1002/dta.281. PMID 21538944.
- Wren BG, Day RO, McLachlan AJ, Williams KM (June 2003). "Pharmacokinetics of estradiol, progesterone, testosterone and dehydroepiandrosterone after transbuccal administration to postmenopausal women". Climacteric. 6 (2): 104–11. doi:10.1080/cmt.6.2.104.111. PMID 12841880.
- Jain SK, Jain A, Gupta Y, Kharya A (February 2008). "Design and development of a mucoadhesive buccal film bearing progesterone". Pharmazie. 63 (2): 129–35. doi:10.1691/ph.2008.7114. PMID 18380399.
- Whitelaw MJ (January 1955). "Effects of Parenteral, Oral, Buccal, and Vaginal Progesterone on the Endometrium". Journal of Clinical Endocrinology & Metabolism. 15 (7): 881–882. ISSN 0021-972X.
- Soule SD, Yanow M (July 1953). "Recovery of pregnanediol from urine following administration of oral anhydrohydroxyprogesterone, buccal progesterone, and intramuscular progesterone". Obstet Gynecol. 2 (1): 68–72. PMID 13073082.
- Maddox DH, Burnside BA, Keith AD (1990). "Buccal delivery of progesterone". Proceedings of the International Symposium on Controlled Release of Bioactive Materials. 17. pp. 291–292.
- Voorspoels J, Comhaire F, De Sy W, Remon JP (1997). "Buccal absorption of progesterone in the dog". Proceedings of the International Symposium on Controlled Release of Bioactive Materials. 24. pp. 185–186.
- Hans Hermann Julius Hager; Walther Kern; Paul Heinz List; Hermann Josef Roth (1969). Hagers Handbuch der Pharmazeutischen Praxis: Für Apotheker, Arzneimittelhersteller, Ärzte und Medizinalbeamte: Wirkstoffgruppen II Chemikalien und Drogen (A-AL). Springer-Verlag. pp. 178–. ISBN 978-3-662-25655-8.
- Ashton Leroy Welsh (1951). Dermatological Formulary: A Guide for Medical Students and Resident Physicians in Dermatology. Educational Publishers. p. 155.
- Chakmakjian ZH, Zachariah NY (June 1987). "Bioavailability of progesterone with different modes of administration". J Reprod Med. 32 (6): 443–8. ISSN 0024-7758. PMID 3612635.
- Villanueva B, Casper RF, Yen SS (April 1981). "Intravaginal administration of progesterone: enhanced absorption after estrogen treatment". Fertil. Steril. 35 (4): 433–7. doi:10.1016/S0015-0282(16)45439-7. PMID 7215569.
- Zygmunt M, Sapa Y (2017). "Progesterone – a new look at an old drug". Reproductive Endocrinology. 0 (33): 17–25. doi:10.18370/2309-4117.2017.33.17-25. ISSN 2411-1295.
[In other pharmacokinetic studies it has been proven that after administration of 100 mg of progesterone the hormone reached its maximum concentration in the plasma after 1-4 hours, which was on average 13.5 ng/mL.]
- Unfer V, Casini ML, Marelli G, Costabile L, Gerli S, Di Renzo GC (2005). "Different routes of progesterone administration and polycystic ovary syndrome: a review of the literature". Gynecol. Endocrinol. 21 (2): 119–27. doi:10.1080/09513590500170049. PMID 16109599.
- Stovall DW, Van Voorhis BJ, Mattingly KL, Sparks AE, Chapler FK, Syrop CH (May 1996). "The effectiveness of sublingual progesterone administration during cryopreserved embryo transfer cycles: results of a matched follow-up study". Fertil. Steril. 65 (5): 986–91. doi:10.1016/S0015-0282(16)58274-0. PMID 8612862.
- Bickers W (August 1949). "Progesterone; a comparison of intramuscular, oral and sublingual routes of administration". J. Clin. Endocrinol. Metab. 9 (8): 736–42. doi:10.1210/jcem-9-8-736. PMID 18133494.
- Corner GW (1944). "The Absorption of Steroid Hormones from the Oral Mucous Membranes, with Special Reference to the Sublingual Administration of Progesterone". American Journal of Obstetrics and Gynecology. 47 (5): 670–677. doi:10.1016/S0002-9378(16)40321-2.
- Greenblatt, Robert B. (1944). "Sublingual Absorption of Progesterone and Anhydrohydroxyprogesterone". The Journal of Clinical Endocrinology & Metabolism. 4 (4): 156–158. doi:10.1210/jcem-4-4-156. ISSN 0021-972X.
- Greenblatt RB (1944). "Perlingual Absorption of Progesterone and Anhydrohydroxyprogesterone". The Journal of Clinical Endocrinology & Metabolism. 4 (7): 321–325. doi:10.1210/jcem-4-7-321. ISSN 0021-972X.
- Steege JF, Rupp SL, Stout AL, Bernhisel M (October 1986). "Bioavailability of nasally administered progesterone". Fertil. Steril. 46 (4): 727–9. doi:10.1016/S0015-0282(16)49661-5. PMID 3758397.
- Posaci C, Smitz J, Camus M, Osmanagaoglu K, Devroey P (June 2000). "Progesterone for the luteal support of assisted reproductive technologies: clinical options". Hum. Reprod. 15 Suppl 1: 129–48. doi:10.1093/humrep/15.suppl_1.129. PMID 10928425.
- Stanczyk FZ (2014). "Treatment of postmenopausal women with topical progesterone creams and gels: are they effective?". Climacteric. 17 Suppl 2: 8–11. doi:10.3109/13697137.2014.944496. PMID 25196424.
- Stanczyk FZ, Paulson RJ, Roy S (2005). "Percutaneous administration of progesterone: blood levels and endometrial protection". Menopause. 12 (2): 232–7. doi:10.1097/00042192-200512020-00019. PMID 15772572.
- Sitruk-Ware R (January 1989). "Transdermal delivery of steroids". Contraception. 39 (1): 1–20. doi:10.1016/0010-7824(89)90012-7. PMID 2642780.
- Bińkowska M, Woroń J (June 2015). "Progestogens in menopausal hormone therapy". Przeglad Menopauzalny = Menopause Review. 14 (2): 134–43. doi:10.5114/pm.2015.52154. PMC 4498031. PMID 26327902.
- https://www.drugs.com/international/progestogel.html
- Marker RE, Krueger J (1940). "Sterols. CXII. Sapogenins. XLI. The Preparation of Trillin and its Conversion to Progesterone". J. Am. Chem. Soc. 62 (12): 3349–3350. doi:10.1021/ja01869a023.
- Zava DT, Dollbaum CM, Blen M (March 1998). "Estrogen and progestin bioactivity of foods, herbs, and spices". Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 217 (3): 369–78. doi:10.3181/00379727-217-44247. PMID 9492350.
- Komesaroff PA, Black CV, Cable V, Sudhir K (June 2001). "Effects of wild yam extract on menopausal symptoms, lipids and sex hormones in healthy menopausal women". Climacteric. 4 (2): 144–50. doi:10.1080/713605087. PMID 11428178.
- Sitruk-Ware, Régine (1993). "Percutaneous and Transdermal Oestrogen Replacement Therapy". Annals of Medicine. 25 (1): 77–82. doi:10.3109/07853899309147862. ISSN 0785-3890.
- Mauvais-Jarvis, Pierre; Sitruk-Ware, Regine; Kuttenn, Frederique (1981). "Benign Breast Disease": 51–94. doi:10.1007/978-1-4615-6571-0_3. Cite journal requires
|journal=(help) - De Boever, J.; Verheugen, C.; Van Maele, G.; Vandekerckhove, D. (1983). "Steroid Concentrations in Serum, Glandular Breast Tissue, and Breast Cyst Fluid of Control and Progesterone-Treated Patients". In Alberto Angeli (ed.). Endocrinology of Cystic Breast Disease (PDF). Raven Press. pp. 93–99.
- Taubert, H.-D. (1989). "Lokaltherapie mit Sexualsteroiden": 214–224. doi:10.1007/978-3-642-50217-0_25. Cite journal requires
|journal=(help) - Wiedersberg S, Guy RH (September 2014). "Transdermal drug delivery: 30+ years of war and still fighting!" (PDF). J Control Release. 190: 150–6. doi:10.1016/j.jconrel.2014.05.022. PMID 24852092.
- Jones H (25 September 2008). Testosterone Deficiency in Men. OUP Oxford. pp. 89–. ISBN 978-0-19-954513-1.
- Rastrelli, G.; Reisman, Y.; Ferri, S.; Prontera, O.; Sforza, A.; Maggi, M.; Corona, G. (2019). "Testosterone Replacement Therapy". Sexual Medicine. pp. 79–93. doi:10.1007/978-981-13-1226-7_8. ISBN 978-981-13-1225-0.
- Potts RO, Lobo RA (May 2005). "Transdermal drug delivery: clinical considerations for the obstetrician-gynecologist". Obstet Gynecol. 105 (5 Pt 1): 953–61. doi:10.1097/01.AOG.0000161958.70059.db. PMID 15863530.
- Du JY, Sanchez P, Kim L, Azen CG, Zava DT, Stanczyk FZ (2013). "Percutaneous progesterone delivery via cream or gel application in postmenopausal women: a randomized cross-over study of progesterone levels in serum, whole blood, saliva, and capillary blood". Menopause. 20 (11): 1169–75. doi:10.1097/GME.0b013e31828d39a2. PMID 23652031.
- Heustess A, Asbill S, Eagerton D, Arnold J (2014). "In vitro skin penetration and skin content of progesterone from various topical formulations". Int J Pharm Compd. 18 (6): 512–5. PMID 25906629.
- Rakel D (2012). Integrative Medicine. Elsevier Health Sciences. pp. 330–. ISBN 1-4377-1793-4.
- De Boever, J.; Desmet, B.; Vandekerckhove, D. (1980). "Variation of progesterone, 20α-dihydroprogesterone and oestradiol concentrations in human mammary tissue and blood after topical administration of progesterone". In P. Mauvais-Jarvis (ed.). Percutaneous absorption of steroids. Academic Press. pp. 259–265.
- Chang KJ, Lee TT, Linares-Cruz G, Fournier S, de Ligniéres B (April 1995). "Influences of percutaneous administration of estradiol and progesterone on human breast epithelial cell cycle in vivo". Fertil. Steril. 63 (4): 785–91. doi:10.1016/S0015-0282(16)57482-2. PMID 7890063.
- Spicer DV, Ursin G, Pike MC (May 1996). "Progesterone concentrations--physiologic or pharmacologic?". Fertil. Steril. 65 (5): 1077–8. doi:10.1016/s0015-0282(16)58295-8. PMID 8612843.
- von Eye Corleta H, Capp E, Ferreira MB (2004). "Pharmacokinetics of natural progesterone vaginal suppository". Gynecol. Obstet. Invest. 58 (2): 105–8. doi:10.1159/000078842. PMID 15192285.
- Rao K, Carp HJ, Fischer R (30 September 2013). Principles & Practice of Assisted Reproductive Technology (3 Vols). JP Medical Ltd. pp. 1025–. ISBN 978-93-5090-736-8.
Vaginal administration of progesterone can take many routes such as micronized vaginal suppositories (e.g. Utrogestan or Cyclogest 600 mg daily), foam release vaginal inserts (Endometrin) or vaginal gels (Crinone 8% gel containing 90 mg micronized progesterone one or two applications daily).
- Racowsky C, Schlegel PN, Fauser BC, Barnard L (7 June 2011). Biennial Review of Infertility. Springer Science & Business Media. pp. 84–85. ISBN 978-1-4419-8456-2.
There are currently three standardized vaginal progesterone formulations commercially available for use in IVF. Micronized progesterone capsules (Utrogestan/Prometrium) are available and widely used in Europe and South America, but not FDA approved for use in IVF/ART in the United States. The two progesterone formulations approved by FDA for use in IVF are Crinone 8% vaginal gel, and more recently – Endometrin 100 mg (natural progesterone in capsules).
- Friend DR (October 2016). "Development of controlled release systems over the past 50years in the area of contraception". J Control Release. 240: 235–241. doi:10.1016/j.jconrel.2015.12.043. PMID 26732558.
- Schmidt JW, Wollner D, Curcio J, Riedlinger J, Kim LS (October 2006). "Hormone replacement therapy in menopausal women: Past problems and future possibilities". Gynecol. Endocrinol. 22 (10): 564–77. doi:10.1080/09513590600927017. PMID 17135036.
- Goletiani NV, Keith DR, Gorsky SJ (October 2007). "Progesterone: review of safety for clinical studies". Exp Clin Psychopharmacol. 15 (5): 427–44. doi:10.1037/1064-1297.15.5.427. PMID 17924777.
- Sills ES (7 September 2015). Screening the Single Euploid Embryo: Molecular Genetics in Reproductive Medicine. Springer. pp. 276–277. ISBN 978-3-319-16892-0.
- Tatford EP (6 December 2012). Problems in Gynaecology. Springer Science & Business Media. pp. 85–. ISBN 978-94-009-4125-0.
Progesterone can also be given by rectal or vaginal suppository (Cyclogest) in a dose of 400 mg daily.
- Aghsa MM, Rahmanpour H, Bagheri M, Davari-Tanha F, Nasr R (October 2012). "A randomized comparison of the efficacy, side effects and patient convenience between vaginal and rectal administration of Cyclogest(®) when used for luteal phase support in ICSI treatment". Arch. Gynecol. Obstet. 286 (4): 1049–54. doi:10.1007/s00404-012-2410-7. PMID 22714063.
- Khrouf M, Slimani S, Khrouf MR, Braham M, Bouyahia M, Berjeb KK, Chaabane HE, Merdassi G, Kaffel AZ, Zhioua A, Zhioua F (2016). "Progesterone for Luteal Phase Support in In Vitro Fertilization: Comparison of Vaginal and Rectal Pessaries to Vaginal Capsules: A Randomized Controlled Study". Clin Med Insights Womens Health. 9: 43–47. doi:10.4137/CMWH.S32156. PMC 5217976. PMID 28096703.
- Tay PY, Lenton EA (June 2005). "The impact of luteal supplement on pregnancy outcome following stimulated IVF cycles" (PDF). Med. J. Malaysia. 60 (2): 151–7. PMID 16114155.
- van der Meer YG, van Loenen AC, Loendersloot EW, Jaszmann LJ (October 1982). "Plasma progesterone levels after using high dose suppositories. A preliminary report". Pharm Weekbl Sci. 4 (5): 135–6. doi:10.1007/BF01959031. PMID 7145597.
- Nillius SJ, Johansson ED (June 1971). "Plasma levels of progesterone after vaginal, rectal, or intramuscular administration of progesterone". Am. J. Obstet. Gynecol. 110 (4): 470–7. doi:10.1016/0002-9378(71)90686-7. PMID 5582003.
- Bernardo-Escudero R, Cortés-Bonilla M, Alonso-Campero R, Francisco-Doce MT, Chavarín-González J, Pimentel-Martínez S, Zambrano-Tapia L (2012). "Observational study of the local tolerability of injectable progesterone microspheres". Gynecol. Obstet. Invest. 73 (2): 124–9. doi:10.1159/000330711. PMID 21997608.
- Ferin J (September 1972). "Effects, Duration of Action and Metabolism in Man". In Tausk M (ed.). Pharmacology of the Endocrine System and Related Drugs: Progesterone, Progestational Drugs and Antifertility Agents. II. Pergamon Press. pp. 13–24. ISBN 978-0080168128. OCLC 278011135.
- Horský J, Presl J (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In J. Horsky, J. Presl (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- Knörr K, Beller FK, Lauritzen C (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 214–. ISBN 978-3-662-00942-0.
- Henzl M, Horský J, Presl J (1965), "Racionální Léčba Gestageny v Gynekologii" [Rational Treatment by Gestagens in Gynecology], Československá gynekologie (in Czech), 30/44 (1/2): 10–16, ISSN 0374-6852,
Rational indications for the use of pure progesterone and some gestagens. These are depot microcrystal drugs (Agolutin depot), Neolutin and the new, perorally effective gestagens (nor-steroids, Superlutin). [...]
- Ober, K. G.; Klein, Irmhild; Weber, Marianne (1954). "Zur Frage einer Progesteronbehandlung: Experimentelle Untersuchungen mit dem Hooker-Forbes-Test und klinische Beobachtungen mit Kristallsuspensionen" [On the question of progesterone treatment: experimental studies with the Hooker-Forbes test and clinical observations with crystal suspensions]. Archiv für Gynäkologie. 184 (5): 543–616. doi:10.1007/BF00976991. ISSN 0003-9128.
- Engelking E (13 March 2013). Der Deutschen Ophthalmologischen Gesellschaft: In München 1950 [The German Ophthalmological Society: In Munich 1950]. Springer-Verlag. pp. 145–146. ISBN 978-3-642-47081-3.
Intramuskulare Injektionen öliger Progesteronlösungen erzielen wohl eine hohe Stoßwirkung, der Dauereffekt ist jedoch nur gering. So kann die Wirkung einer Injektion von 10 mg [intramuskulär] in schweren Glaukomfällen schon nach etwa 6 Stunden vergehen. Das Ergebnis ist dann ein Wiederhochschnellen des Augendruckes. Um den Hormonspiegel nicht zu rasch wieder abfallen zu lassen und gleichzeitig eine Stoßwirkung zu erzielen, mischen wir aus diesem Grunde gerne die ölige Lösung mit einer Kristallsuspension. Wir injizierten meist in den Deltoideus. Bei Hormonkristallsuspensionen braucht man ziemlich dicke Nadeln und benützt am besten eine 1 ccm Spritze. Für die augenärztliche Praxis ist letztere Art der Depotbehandlung am zweckmäßigsten. In schwereren Fällen mit chronischer hoher Drucksteigerung wirken [zum Beispiel] bei Verwendung von [Ampulle] mit je 30 mg [Kristallsuspension] die ersten Injektionen nur wenige Tage; mit zunehmender Dauer der Behandlung kann man dann jedoch die Zeitabstände zswischen den einzelnen Spritzen bis auf mehrere Wochen erhöhen. Die meisten Patienten spuren selbst deutlich, wenn die Depotwirkung nachläßt und kommen spontan, sich eine neue Injektion zu holen. [...] Depot: Flavolutan (Boehringer & Söhne) Kristallsuspension amp a 30 mg.
- Shearman RP (June 1975). "The development of depot contraceptives". J. Steroid Biochem. 6 (6): 899–902. doi:10.1016/0022-4731(75)90323-4. PMID 1177432.
- Edkins, Robert Patrick (1959). "The Modification of the Duration of Drug Action". Journal of Pharmacy and Pharmacology. 11 (S1): 54T–66T. doi:10.1111/j.2042-7158.1959.tb10412.x. ISSN 0022-3573.
- Lens J, Overbeek GA, Polderman J (1949). "The effect of sex hormones in some organic solvents; emulsified in water". Acta Endocrinol. 2 (4): 396–404. doi:10.1530/acta.0.0020396. PMID 18140399.
- Herrmann, U. (1958). "Abhängigkeit der durch Oestrogen- und Progesteron-Kristalle induzierten Abbruchblutung von der Korngröße". Gynecologic and Obstetric Investigation. 146 (4): 318–323. doi:10.1159/000306607. ISSN 1423-002X.
- Kahr H (8 March 2013). Konservative Therapie der Frauenkrankheiten: Anzeigen, Grenzen und Methoden Einschliesslich der Rezeptur. Springer-Verlag. pp. 20–21. ISBN 978-3-7091-5694-0.
- https://web.archive.org/web/20190519061250/http://www.sukl.cz/download/spc/SPC14550.pdf
- Články v českých časopisech. Statni knihovna ČSSR. 1960. pp. 1601, 1642.
- Plzensky lekarsky sbornik. Supplementum. 1961. p. 169.
- Review of Czechoslovak Medicine. Avicenum - Czechoslovak Medical Press. 1973. p. 5.
Progesterone, aqueous microcrystalline suspension (Agolutin Depot SPOFA)
- Ufer, Joachim (1968). "Die therapeutische Anwendung der Gestagene beim Menschen" [Therapeutic Use of Progestagens in Humans]. Die Gestagene [Progestogens]. Springer-Verlag. pp. 1026–1124. doi:10.1007/978-3-642-99941-3_7. ISBN 978-3-642-99941-3.
C. Dysfunktionelle Uterusblutungen. [...] 1. Depotinjektionen. 1. Originalmethode nach KAUFMANN und OBER. Es wird 1 Amp. mit 200 mg Progesteron und 10 mg Oestradiol-Monobenzoat als Kristallsuspension (Sistocyclin) injiziert [676, 678, 679, 295, 482, 365, 434, 563, 400]. [...] Beispiele. KAUFMANN et al. [485]: 400 mg Progesteron + 20 mg Oestradiolmonobenzoat Kristallsuspension. ELERT [224] U. HERRMANN [363]: 200 mg Progesteron + 10 mg Oestradiolmono benzoat Kristallsuspension.
- Ciba Symposium. Ciba. 1957.
CIBA's range of hormone preparations has been increased with the advent of "Sistocyclin", one ampoule of which contains 200 mg progesterone and 10 mg oestradiol monobenzoate in crystalline suspension; it thus meets the requirements—in line with the most recent findings of the KAUFMANN Clinic—of cases marked by deficiency of corpus luteum hormone, e. g. in functional bleeding such as metropathia haemorrhagica.
- Ciba Zeitschrift. 1957. p. 3001.
Sistocyclin - a microcrystal suspension containing 200 mg progesterone and 10 mg oestradiol monobenzoate per ampoule - has become particularly useful in the treatment of so-called, functional [...]
- Willibald Pschyrembel (1968). Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. pp. 601–. ISBN 978-3-11-150424-7.
- Joachim Ufer (1960). Hormontherapie in der Frauenheilkunde: Grundlagen und Praxis. Gruyter. p. 153.
- Willibald Pschyrembel (15 June 2011). Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. pp. 601–. ISBN 978-3-11-150424-7.
- SWYER GI (January 1960). "Progestogens and their clinical uses: Part I". Br Med J. 1 (5165): 48–9. doi:10.1136/bmj.1.5165.48. PMC 1966392. PMID 13836156.
- Janet Brotherton (1976). Sex Hormone Pharmacology. Academic Press. p. 38. ISBN 978-0-12-137250-7.
- Ian S. Fraser (1998). Estrogens and Progestogens in Clinical Practice. Churchill Livingstone. pp. 13, 92. ISBN 978-0-443-04706-0.
- Mishell DR (May 1996). "Pharmacokinetics of depot medroxyprogesterone acetate contraception". The Journal of Reproductive Medicine. 41 (5 Suppl): 381–90. PMID 8725700.
- Goebelsmann U (1986). "Pharmacokinetics of Contraceptive Steroids in Humans". In Gregoire AT, Blye RP (eds.). Contraceptive Steroids: Pharmacology and Safety. Springer Science & Business Media. pp. 67–111. doi:10.1007/978-1-4613-2241-2_4. ISBN 978-1-4613-2241-2.
- Tobías GM, Usabiaga RA, Riaño JD, Espinoza AB, Tovar SR, Martínez ER (2008). "Progesterona en Microesferas para el Tratamiento en Infertilidad" (PDF). Acta Médica Grupo Ángeles. 6 (1): 8.
- Tobías GM, Martínez ER (2009). "Técnica para Aplicación de Fármacos con Microesferas en Suspensión" (PDF). Acta Médica Grupo Ángeles. 7 (1): 13.
- "Juvenum (estradiol/progesterone) - Medicamentos PLM". Medicamentos PLM. Archived from the original on 2018-09-17. Retrieved 2018-09-17.
- http://adisinsight.springer.com/drugs/800044558
- https://www.drugs.com/international/juvenum.html
- Cortés-Bonilla M, Alonso-Campero R, Bernardo-Escudero R, Francisco-Doce MT, Chavarín-González J, Pérez-Cuevas R, Chedraui P (October 2016). "Improvement of quality of life and menopausal symptoms in climacteric women treated with low-dose monthly parenteral formulations of non-polymeric microspheres of 17β-estradiol/progesterone". Gynecol. Endocrinol. 32 (10): 831–834. doi:10.1080/09513590.2016.1183628. PMID 27187320.
- Cortés-Bonilla M, Bernardo-Escudero R, Alonso-Campero R, Francisco-Doce MT, Hernández-Valencia M, Celis-González C, Márquez-Oñate R, Chedraui P, Uribe JA (July 2015). "Treatment of menopausal symptoms with three low-dose continuous sequential 17β-estradiol/progesterone parenteral monthly formulations using novel non-polymeric microsphere technology". Gynecol. Endocrinol. 31 (7): 552–9. doi:10.3109/09513590.2015.1019853. PMC 4776687. PMID 26062108.
- Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- Bagade O, Pawar V, Patel R, Patel B, Awasarkar V, Diwate S (2014). "Increasing use of long-acting reversible contraception: safe, reliable, and cost-effective birth control" (PDF). World J Pharm Pharm Sci. 3 (10): 364–392. ISSN 2278-4357.
- Newton JR, D'arcangues C, Hall PE (1994). "A review of "once-a-month" combined injectable contraceptives". J Obstet Gynaecol (Lahore). 4 Suppl 1: S1–34. doi:10.3109/01443619409027641. PMID 12290848.
- Garza-Flores J, Fatinikun T, Hernandez L, Ramos I, Cardenas M, Menjivar M (July 1991). "A pilot study on the assessment of a progesterone/estradiol sustained release as once-a-month-injectable contraceptive". Contraception. 44 (1): 45–59. doi:10.1016/0010-7824(91)90105-O. PMID 1893701.
- Garza-Flores J, Hall PE, Perez-Palacios G (1991). "Long-acting hormonal contraceptives for women". J. Steroid Biochem. Mol. Biol. 40 (4–6): 697–704. doi:10.1016/0960-0760(91)90293-E. PMID 1958567.
- Lockwood G, Griesinger G, Cometti B (2014). "Subcutaneous progesterone versus vaginal progesterone gel for luteal phase support in in vitro fertilization: a noninferiority randomized controlled study". Fertil. Steril. 101 (1): 112–119.e3. doi:10.1016/j.fertnstert.2013.09.010. PMID 24140033.
- Baker VL, Jones CA, Doody K, Foulk R, Yee B, Adamson GD, Cometti B, DeVane G, Hubert G, Trevisan S, Hoehler F, Jones C, Soules M (2014). "A randomized, controlled trial comparing the efficacy and safety of aqueous subcutaneous progesterone with vaginal progesterone for luteal phase support of in vitro fertilization". Hum. Reprod. 29 (10): 2212–20. doi:10.1093/humrep/deu194. PMC 4164149. PMID 25100106.
- Kimmig J (1962). Therapie der Haut- und Geschlechtskrankheiten. Springer-Verlag. pp. 507–. ISBN 978-3-642-94850-3.
- Lobo RA, Kelsey J, Marcus R (22 May 2000). Menopause: Biology and Pathobiology. Academic Press. pp. 619–. ISBN 978-0-08-053620-0.
- https://www.drugs.com/international/progesterone.html
- Pinkerton JV, Pickar JH (February 2016). "Update on medical and regulatory issues pertaining to compounded and FDA-approved drugs, including hormone therapy". Menopause. 23 (2): 215–23. doi:10.1097/GME.0000000000000523. PMC 4927324. PMID 26418479.
- Santoro N, Braunstein GD, Butts CL, Martin KA, McDermott M, Pinkerton JV (April 2016). "Compounded Bioidentical Hormones in Endocrinology Practice: An Endocrine Society Scientific Statement". J. Clin. Endocrinol. Metab. 101 (4): 1318–43. doi:10.1210/jc.2016-1271. PMID 27032319.
- Pinkerton JV (December 2014). "What are the concerns about custom-compounded "bioidentical" hormone therapy?". Menopause. 21 (12): 1298–300. doi:10.1097/GME.0000000000000376. PMID 25387347.
- Mishell DR (1941). "A clinical study of progesterone therapy by pellet implantation". American Journal of Obstetrics and Gynecology. 41 (4): 687–693. doi:10.1016/S0002-9378(41)90665-8. ISSN 0002-9378.
- Bishop PM (1944). "Endocrine Therapy in Gynaecology and Obstetrics". BJOG: An International Journal of Obstetrics and Gynaecology. 51 (1): 51–63. doi:10.1111/j.1471-0528.1944.tb07317.x. ISSN 1470-0328.
- Foss GL (1943). "Implantation of Sex Hormone Tablets in Man". British Medical Bulletin. 1 (2): 21–22. doi:10.1093/oxfordjournals.bmb.a070135. ISSN 1471-8391.
- Greenblatt RB, Hair LQ (1945). "Absorption of Pellets of Progesterone". The Journal of Clinical Endocrinology & Metabolism. 5 (1): 38–39. doi:10.1210/jcem-5-1-38.
- Greenblatt RB, Suran RR (February 1949). "Indications for hormonal pellets in the therapy of endocrine and gynecic disorders". Am. J. Obstet. Gynecol. 57 (2): 294–301. doi:10.1016/0002-9378(49)90429-9. PMID 18123090.
- Bishop PM, Richards NA, Doll R (July 1950). "Habitual abortion; prophylactic value of progesterone pellet implantation". Br Med J. 2 (4671): 130–3. doi:10.1136/bmj.2.4671.130. PMC 2038208. PMID 15434335.
- Bishop PM, Folley SJ (August 1951). "Absorption of hormone implants in man". Lancet. 2 (6676): 229–32. doi:10.1016/S0140-6736(51)93237-0. PMID 14862159.
- Bishop PM, Richards NA (February 1952). "Habitual abortion; further observations on the prophylactic value of progesterone pellet implantation". Br Med J. 1 (4752): 244–6. doi:10.1136/bmj.1.4752.244. PMC 2022587. PMID 14896102.
- Scow RO, Ebling FJ, Henderson IW (1973). Endocrinology: Proceedings of the Fourth International Congress of Endocrinology, Washington, D.C., June 18-24, 1972. Excerpta Medica. p. 978. ISBN 978-90-219-0166-4.
Subdermal implants. Another way to obtain prolonged activity is by using subcutaneous implants made by compressing crystalline hormones. However, progesterone pellets are poorly tolerated and sterile abscesses or extrusion may occur in about 15—20% of cases (Greenblatt, personal communication, 1969). For this reason it appears that implants made of pure progestational steroids have little chance of finding a place in contraceptive therapy. [...]
- Croxatto HB, Díaz S (1987). "The place of progesterone in human contraception". J. Steroid Biochem. 27 (4–6): 991–4. doi:10.1016/0022-4731(87)90179-8. PMID 3320572.
- Shaaban MM (1991). "Contraception with progestogens and progesterone during lactation". J. Steroid Biochem. Mol. Biol. 40 (4–6): 705–10. doi:10.1016/0960-0760(91)90294-F. PMID 1835650.
- Croxatto HB, Díaz S, Peralta O, Juez G, Casado ME, Salvatierra AM, Durán E (September 1982). "Fertility regulation in nursing women. II. Comparative performance of progesterone implants versus placebo and copper T". Am. J. Obstet. Gynecol. 144 (2): 201–8. doi:10.1016/0002-9378(82)90628-7. PMID 7114130.
- Díaz S, Peralta O, Juez G, Herreros C, Casado ME, Salvatierra AM, Miranda P, Croxatto HB (October 1984). "Fertility regulation in nursing women. VI. Contraceptive effectiveness of a subdermal progesterone implant". Contraception. 30 (4): 311–25. doi:10.1016/S0010-7824(84)80023-2. PMID 6509984.
- Mannino CA, South SM, Inturrisi CE, Quinones-Jenab V (December 2005). "Pharmacokinetics and effects of 17beta-estradiol and progesterone implants in ovariectomized rats". J Pain. 6 (12): 809–16. doi:10.1016/j.jpain.2005.07.007. PMID 16326369.
- Benagiano G, Gabelnick H, Farris M (September 2008). "Contraceptive devices: subcutaneous delivery systems". Expert Rev Med Devices. 5 (5): 623–37. doi:10.1586/17434440.5.5.623. PMID 18803473.
- Freeman S, Shulman LP (February 2010). "Considerations for the use of progestin-only contraceptives". J Am Acad Nurse Pract. 22 (2): 81–91. doi:10.1111/j.1745-7599.2009.00473.x. PMID 20132366.
- Falcone T, Hurd WW (1 January 2007). Clinical Reproductive Medicine and Surgery. Elsevier Health Sciences. pp. 406–. ISBN 0-323-03309-1.
- Sloane E (2002). Biology of Women. Cengage Learning. pp. 445–. ISBN 0-7668-1142-5.
- Aiken AR, Trussell J (2014). "Recent advances in contraception". F1000Prime Rep. 6: 113. doi:10.12703/P6-113. PMC 4251416. PMID 25580267.
- Lobo RA (5 June 2007). Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Elsevier. pp. 210–. ISBN 978-0-08-055309-2.
- Sitruk-Ware R, Bricaire C, De Lignieres B, Yaneva H, Mauvais-Jarvis P (October 1987). "Oral micronized progesterone. Bioavailability pharmacokinetics, pharmacological and therapeutic implications--a review". Contraception. 36 (4): 373–402. doi:10.1016/0010-7824(87)90088-6. PMID 3327648.
- Simon JA (December 1995). "Micronized progesterone: vaginal and oral uses". Clinical Obstetrics and Gynecology. 38 (4): 902–14. doi:10.1097/00003081-199538040-00024. PMID 8616985.
- Stanczyk FZ, Hapgood JP, Winer S, Mishell DR (April 2013). "Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects". Endocr. Rev. 34 (2): 171–208. doi:10.1210/er.2012-1008. PMC 3610676. PMID 23238854.
Further reading
- Langecker H (1968). "Resorption, Verteilung und Ausscheidung der Gestagene" [Absorption, Distribution, and Excretion of Progestogens]. Die Gestagene [The Progestogens]. Springer-Verlag. pp. 264–351. doi:10.1007/978-3-642-99941-3_3. ISBN 978-3-642-99941-3.
- Sitruk-Ware R, Bricaire C, De Lignieres B, Yaneva H, Mauvais-Jarvis P (October 1987). "Oral micronized progesterone. Bioavailability pharmacokinetics, pharmacological and therapeutic implications--a review". Contraception. 36 (4): 373–402. doi:10.1016/0010-7824(87)90088-6. PMID 3327648.
- Simon JA (December 1995). "Micronized progesterone: vaginal and oral uses". Clinical Obstetrics and Gynecology. 38 (4): 902–14. doi:10.1097/00003081-199538040-00024. PMID 8616985.
- Ruan X, Mueck AO (November 2014). "Systemic progesterone therapy--oral, vaginal, injections and even transdermal?". Maturitas. 79 (3): 248–55. doi:10.1016/j.maturitas.2014.07.009. PMID 25113944.