Medroxyprogesterone
Medroxyprogesterone (MP), is a progestin which is not used medically.[1][2][3][4] A derivative, medroxyprogesterone acetate (MPA), is used as a medication in humans, and is far more widely known in comparison.[5] Medroxyprogesterone is sometimes used as a synonym for medroxyprogesterone acetate,[5] and what is almost always being referred to when the term is used is MPA and not medroxyprogesterone.[6]
 | |
 | |
| Clinical data | |
|---|---|
| Other names | MP; Methylhydroxyprogesterone; 6α-Methyl-17α-hydroxyprogesterone; 6α-Methyl-17α-hydroxypregn-4-en-3,20-dione |
| Drug class | Progestin; Progestogen |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.545 |
| Chemical and physical data | |
| Formula | C22H32O3 |
| Molar mass | 344.488 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Pharmacology
Pharmacodynamics
Compared to MPA, medroxyprogesterone is over two orders of magnitude less potent as a progestogen.[7] Medroxyprogesterone is also notable in that it is a minor metabolite of MPA.[8] In addition to its progestagenic activity, medroxyprogesterone is a weak antiandrogen in vitro on human androgen receptor.[9]
| Compound | Ki | EC50a | EC50b |
|---|---|---|---|
| Progesterone | 4.3 | 0.9 | 25 |
| Medroxyprogesterone | 241 | 47 | 32 |
| Medroxyprogesterone acetate | 1.2 | 0.6 | 0.15 |
| Values are nM. a = Coactivator recruitment. b = Reporter cell line. | |||
Chemistry
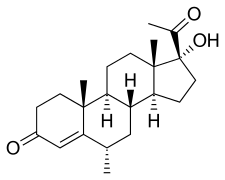
Medroxyprogesterone, also known as 6α-methyl-17α-hydroxyprogesterone or as 6α-methyl-17α-hydroxypregn-4-en-3,20-dione, is a synthetic pregnane steroid and a derivative of progesterone.[1][2] It is specifically a derivative of 17α-hydroxyprogesterone with a methyl group at the C6α position.[1][2] The generic name of medroxyprogesterone is a contraction of 6α-methyl-17α-hydroxyprogesterone. It is closely related to medrogestone as well as other unesterified 17α-hydroxyprogesterone derivatives such as chlormadinone, cyproterone, and megestrol.[1][2]
Society and culture
References
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 657–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 638–. ISBN 978-3-88763-075-1.
- Morton IK, Hall JM (31 October 1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 173–. ISBN 978-0-7514-0499-9.
- "Medroxyprogesterone".
- "MedroxyPROGESTERone: Drug Information Provided by Lexi-Comp". Merck Manual. 2009-12-01. Retrieved 2010-07-08.
- Lenco W, Mcknight M, Macdonald AS (January 1975). "Effects of cortisone acetate, methylprednisolone and medroxyprogesterone on wound contracture and epithelization in rabbits". Annals of Surgery. 181 (1): 67–73. doi:10.1097/00000658-197501000-00015. PMC 1343717. PMID 1119869.
- Pullen MA, Laping N, Edwards R, Bray J (September 2006). "Determination of conformational changes in the progesterone receptor using ELISA-like assays". Steroids. 71 (9): 792–8. doi:10.1016/j.steroids.2006.05.009. PMID 16784762.
- Ishihara M, Kirdani Y, Osawa Y, Sandberg AA (January 1976). "The metabolic fate of medroxyprogesterone acetate in the baboon". Journal of Steroid Biochemistry. 7 (1): 65–70. doi:10.1016/0022-4731(76)90167-9. PMID 1271819.
- Šauer P, Bořík A, Golovko O, Grabic R, Staňová AV, Valentová O, et al. (November 2018). "Do progestins contribute to (anti-)androgenic activities in aquatic environments?". Environmental Pollution. 242 (Pt A): 417–425. doi:10.1016/j.envpol.2018.06.104. PMID 29990947.