Levomethadone
Levomethadone, sold under the brand name L-Polamidon among others, is a synthetic opioid analgesic and antitussive which is marketed in Europe and is used for pain management and in opioid maintenance therapy.[1][2][3] In addition to being used as a pharmaceutical drug itself, levomethadone is the main therapeutic component of methadone.[2]
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Levamethadone; l-Methadone; 6R-Methadone; (–)-Methadone; R-(–)-Methadone; D-(–)-Methadone |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth, IV, IM, SC, IT[1] |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | High[1] |
| Protein binding | 60–90%[1] |
| Elimination half-life | ~18 hours[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.120.592 |
| Chemical and physical data | |
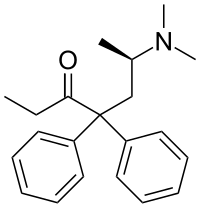
| Formula | C21H27NO |
| Molar mass | 309.445 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 99.5 °C (211.1 °F) |
| Solubility in water | 48.48 mg/mL (20 °C) |
SMILES
| |
InChI
| |
Where it is used, levomethadone is used for narcotic maintenance in place of, or in some cases alongside as an alternative, to racemic methadone, owing to concern about the cardiotoxic and QT-prolonging action of racemic methadone being exclusively caused by the dextrorotatory enantiomer, dextromethadone.
Pharmacology
Pharmacodynamics
Levomethadone has approximately 50x the potency of the S-(+)-enantiomer as well as greater μ-opioid receptor selectivity.[1][4] Accordingly, it is about twice as potent as methadone by weight and its effects are virtually identical in comparison.[5][6] In addition to its activity at the opioid receptors, levomethadone has been found to act as a weak competitive antagonist of the N-methyl-D-aspartate (NMDA) receptor complex[7] and as a potent noncompetitive antagonist of the α3β4 nicotinic acetylcholine (nACh) receptor.[8]
| Compound | Affinities (Ki) | Ratios | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MOR | DOR | KOR | SERT | NET | NMDAR | MOR:DOR:KOR | SERT:NET | ||
| Methadone | 1.7 nM | 435 nM | 405 nM | ND | ND | 2,500–8,300 nM | 1:256:238 | ND | |
| Dextromethadone | 19.7 nM | 960 nM | 1,370 nM | 992 nM | 12,700 nM | 2,600–7,400 nM | 1:49:70 | 1:13 | |
| Levomethadone | 0.945 nM | 371 nM | 1,860 nM | 14.1 nM | 702 nM | 2,800–3,400 nM | 1:393:1968 | 1:50 | |
| Sources: See template. | |||||||||
Chemistry
The separation of the stereoisomers is one of the easier in organic chemistry and is described in the original patent. It involves "treatment of racemic methadone base with d-(+)-tartaric acid in an acetone/water mixture [which] precipitates almost solely the dextro-methadone levo-tartrate, and the more potent levo-methadone can easily be retrieved from the mother liquor in a high state of optical purity" [9]
There is now an asymmetric synthesis[10] available to prepare both levomethadone (R-(−)-methadone) and dextromethadone (S-(+)-methadone).[11]
Society and culture
Brand names
Levomethadone has been sold under brand names including L-Polaflux, L-Polamidon, L-Polamivet, Levadone, Levo-Methasan, Levothyl, Mevodict, and Vistadict, among others.[12][3][2]
Legal status
Levomethadone is listed under the Single Convention On Narcotic Drugs 1961 and is a Schedule II Narcotic controlled substance in the US as an isomer of methadone (ACSCN 9250) and is not listed separately, nor is dextromethadone.[13] It is similarly controlled under the German Betäubungsmittelgesetz and similar laws in practically every other country.
References
- Helmut Buschmann (20 December 2002). Analgesics: From Chemistry and Pharmacology to Clinical Application. Wiley-VCH. p. 196. ISBN 978-3-527-30403-5. Retrieved 17 May 2012.
- F.. Macdonald (1997). Dictionary of Pharmacological Agents. CRC Press. p. 1294. ISBN 978-0-412-46630-4. Retrieved 17 May 2012.
- Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 605. ISBN 978-3-88763-075-1. Retrieved 17 May 2012.
- Renate Förch; Holger Schönherr; A. Tobias A. Jenkins (11 August 2009). Surface Design: Applications in Bioscience and Nanotechnology. Wiley-VCH. p. 193. ISBN 978-3-527-40789-7. Retrieved 17 May 2012.
- Eduardo Bruera; Sriram Yennurajalingam (16 August 2011). Oxford American Handbook of Hospice and Palliative Medicine. Oxford University Press. p. 43. ISBN 978-0-19-538015-6. Retrieved 17 May 2012.
- Verthein U, Ullmann R, Lachmann A, et al. (November 2005). "The effects of racemic D,L-methadone and L-methadone in substituted patients--a randomized controlled study". Drug and Alcohol Dependence. 80 (2): 267–71. doi:10.1016/j.drugalcdep.2005.04.007. PMID 15916866.
- Eric C. Strain; Maxine L. Stitzer (4 November 2005). The Treatment of Opioid Dependence. JHU Press. p. 63. ISBN 978-0-8018-8303-3. Retrieved 19 May 2012.
- Xiao Y, Smith RD, Caruso FS, Kellar KJ (October 2001). "Blockade of rat alpha3beta4 nicotinic receptor function by methadone, its metabolites, and structural analogs". The Journal of Pharmacology and Experimental Therapeutics. 299 (1): 366–71. PMID 11561100.
- https://www.erowid.org/archive/rhodium/chemistry/methadone.html
- Hull JD, Scheinmann F, Turner NJ (March 2003). "Synthesis of optically active methadones, LAAM and bufuralol by lipase-catalysed acylations". Tetrahedron: Asymmetry. 14 (5): 567–576. doi:10.1016/S0957-4166(03)00019-3.
- US patent 6143933
- https://www.drugs.com/international/levomethadone.html
- http://www.deadiversion.usdoj.gov/quotas/conv_factor/index.html