Dimethandrolone
Dimethandrolone (DMA), also known by its developmental code name CDB-1321, is an experimental androgen/anabolic steroid (AAS) and progestogen medication which is under investigation for potential clinical use.[1][2][3]
 | |
| Clinical data | |
|---|---|
| Other names | CDB-1321; Dimethylnandrolone; 7α,11β-Dimethyl-19-nortestosterone; 7α,11β-Dimethylestr-4-en-17β-ol-3-one; 7α,11β-Dimethyl-19-norandrost-4-en-17β-ol-3-one |
| Routes of administration | By mouth |
| Drug class | Androgen; Anabolic steroid; Progestogen |
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C20H30O2 |
| Molar mass | 302.451 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Dimethandrolone is an AAS, and hence is an agonist of the androgen receptor, the biological target of androgens like testosterone.[1] It is also a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[1] Due to its androgenic and progestogenic activity, dimethandrolone has antigonadotropic effects.[1] It has no estrogenic activity.[1][4]
Dimethandrolone was first described in 1997.[5] It was developed by the Contraceptive Development Branch of the National Institute of Child Health and Human Development, an agency in the United States government.[1][6]
An ester and prodrug of dimethandrolone, dimethandrolone undecanoate (DMAU) (CDB-4521), is under development for potential use as a birth control pill for men and in androgen replacement therapy for men.[1][2][3][7]
Side effects
Pharmacology
Pharmacodynamics
Dimethandrolone is an AAS, though it has also been described as a selective androgen receptor modulator (SARM).[1][2][3] As an AAS, it is a potent agonist of the androgen receptor (AR).[1][2]
Unlike testosterone and various other AAS, dimethandrolone is not metabolized by 5α-reductase.[2] In addition, the 5α-reduced derivative of dimethandrolone, 5α-dihydrodimethandrolone (5α-DHDMA), possesses only 30 to 40% of the potency of dimethandrolone as an agonist of the AR, indicating that dimethandrolone does not require potentiation by 5α-reductase for its activity as an AAS and that even if it were a substrate for 5α-reductase, it would not be potentiated in androgenic tissues like the skin and prostate.[2] As such, dimethandrolone and ester prodrugs of it like DMAU are thought to have a reduced risk of androgenic side effects and conditions such as benign prostatic hyperplasia, prostate cancer, pattern scalp hair loss, and acne relative to testosterone and certain other AAS.[2]
Dimethandrolone is not a substrate for aromatase, and for this reason, is not converted into the corresponding aromatic A-ring derivative 7α,11β-dimethylestradiol, a potent estrogen.[3][4] As such, dimethandrolone is not estrogenic.[4] This is in contrast to nandrolone, which, although its rate of aromatization into the estrogen estradiol is reduced relative to that of testosterone, is still converted to a significant extent.[4]
Similarly to nandrolone and other 19-nortestosterone derivatives, dimethandrolone is a potent progestogen in addition to AAS.[1] This property may serve to augment its antigonadotropic activity, which in turn may improve its effectiveness as an antispermatogenic agent and male contraceptive.[1] This is salient and potentially beneficial as male contraceptives based on androgens alone have failed to produce satisfactory azoospermia in around one-third of men.[1]
Dimethandrolone has shown minimal potential for hepatotoxicity in animal studies, which is in accordance with the fact that it is not a 17α-alkylated AAS.[6]
Chemistry
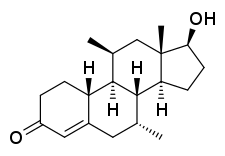
Dimethandrolone, also known as 7α,11β-dimethyl-19-nortestosterone or as 7α,11β-dimethylestr-4-en-17β-ol-3-one, is a synthetic estrane steroid and a non-17α-alkylated derivative of nandrolone (19-nortestosterone).[1]
Esters
Aside from the C17β undecanoate ester of dimethandrolone, DMAU (CDB-4521),[1][2][3] a few other esters, such as dimethandrolone buciclate (CDB-4386A) and dimethandrolone dodecylcarbonate (CDB-4730), have also been developed.[8][9]
Analogues
Other AAS that are closely related to dimethandrolone (besides nandrolone) include trestolone (also known as 7α-methyl-19-nortestosterone (MENT)) and 11β-methyl-19-nortestosterone (11β-MNT) and their respective C17β esters trestolone acetate and 11β-MNT dodecylcarbonate (11β-MNTDC).[1][2]
History
A patent for dimethandrolone was filed in 1997 and was granted in 1999.[5] Subsequently, a patent for DMAU and dimethandrolone buciclate was filed in 2002 and was granted to the United States government in 2003.[10] Dimethandrolone was developed under the code name CDB-1321 by the Contraceptive Development Branch of the National Institute of Child Health and Human Development, one of the National Institutes of Health in the United States Department of Health and Human Services.[1][6]
References
- Attardi, Barbara J.; Hild, Sheri A.; Reel, Jerry R. (June 2006). "Dimethandrolone Undecanoate: A New Potent Orally Active Androgen with Progestational Activity". Endocrinology. 147 (6): 3016–3026. doi:10.1210/en.2005-1524. ISSN 0013-7227. PMID 16497801.
- Attardi, Barbara J.; Hild, Sheri A.; Koduri, Sailaja; Pham, Trung; Pessaint, Laurent; Engbring, Jean; Till, Bruce; Gropp, David; Semon, Anne; Reel, Jerry R. (October 2010). "The potent synthetic androgens, dimethandrolone (7α,11β-dimethyl-19-nortestosterone) and 11β-methyl-19-nortestosterone, do not require 5α-reduction to exert their maximal androgenic effects". The Journal of Steroid Biochemistry and Molecular Biology. 122 (4): 212–218. doi:10.1016/j.jsbmb.2010.06.009. PMC 2949447. PMID 20599615.
- Wang, Christina; Swerdloff, Ronald S. (November 2010). "Hormonal Approaches to Male contraception". Current Opinion in Urology. 20 (6): 520–524. doi:10.1097/MOU.0b013e32833f1b4a. ISSN 0963-0643. PMC 3078035. PMID 20808223.
- Attardi, Barbara J.; Pham, Trung C.; Radler, Lisa M.; Burgenson, Janet; Hild, Sheri A.; Reel, Jerry R. (June 2008). "Dimethandrolone (7α,11β-dimethyl-19-nortestosterone) and 11β-methyl-19-nortestosterone are not converted to aromatic A-ring products in the presence of recombinant human aromatase". The Journal of Steroid Biochemistry and Molecular Biology. 110 (3–5): 214–222. doi:10.1016/j.jsbmb.2007.11.009. PMC 2575079. PMID 18555683.
- Cook, C. E., Kepler, J. A., Lee, Y. W., & Wani, M. C. (1999). U.S. Patent No. 5,952,319. Washington, DC: U.S. Patent and Trademark Office. https://patents.google.com/patent/US5952319A/en
- Attardi, Barbara J.; Engbring, Jean A.; Gropp, David; Hild, Sheri Ann (September–October 2011). "Development of Dimethandrolone 17β-Undecanoate (DMAU) as an Oral Male Hormonal Contraceptive: Induction of Infertility and Recovery of Fertility in Adult Male Rabbits". Journal of Andrology. 32 (5): 530–540. doi:10.2164/jandrol.110.011817. ISSN 1939-4640. PMID 21164142.
- "Dimethandrolone undecanoate shows promise as a male birth control pill | Endocrine Society".
- Blye, Richard, and Hyun Kim. "Methods of making and using 7a, 11b-dimethyl-17b-hydroxy-4-estren-3-one 17b-trans-4-n-butylcyclohexane carboxylate and 7a, 11b-dimethyl-17b-hydroxyestr-4-en-3-one 17-undecanoate." U.S. Patent Application No. 10/260,854.
- Blye, Richard P., and Hyun K. Kim. "Nandrolone 17β-carbonates." U.S. Patent No. 7,820,642. 26 Oct. 2010.
- Blye, Richard, and Hyun Kim. "Methods of making and using 7a, 11b-dimethyl-17b-hydroxy-4-estren-3-one 17b-trans-4-n-butylcyclohexane carboxylate and 7a, 11b-dimethyl-17b-hydroxyestr-4-en-3-one 17-undecanoate." U.S. Patent Application 10/260,854, filed April 10, 2003. https://patents.google.com/patent/US20030069215A1/en