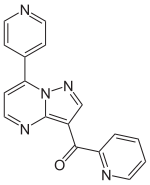
Ocinaplon
Ocinaplon is an anxiolytic drug in the pyrazolopyrimidine family of drugs. Other pyrazolopyrimidine drugs include zaleplon and indiplon.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H11N5O |
| Molar mass | 301.302 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Ocinaplon has a similar pharmacological profile to the benzodiazepine family of drugs, but with mainly anxiolytic properties and relatively little sedative or amnestic effect.[1]
Medical uses
A 2019 review found tentative evidence of benefit in anxiety.[2]
Mechanism of action
The mechanism of action by which ocinaplon produces its anxiolytic effects is by modulating GABAA receptors,[3] although ocinaplon is more subtype-selective than most benzodiazepines.[4]
Availability
Development of ocinaplon is discontinued due to liver complications that occurred in one of the Phase III subjects.[5]
Synthesis
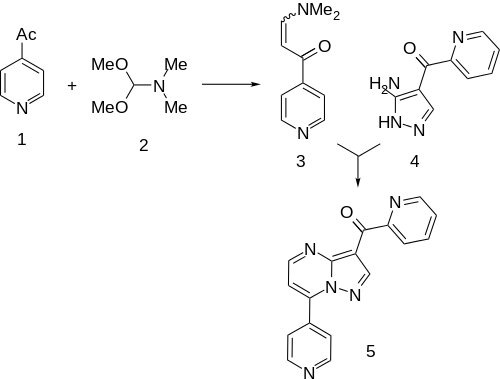
Condensation of 4-Acetylpyridine[8] with N,N-Dimethylformamide dimethyl acetal (DMFDMA) gives the "enamide" (3). This is then condensed with (3-Amino-1H-pyrazol-4-yl)(2-pyridinyl)methanone (4) (96219-90-8).[9][10] This is the same intermediate as was used in the synthesis of zaleplon in which the nitrile is replaced by a 2-acetylpyridil moiety. This affords the anxiolytic agent ocinaplon (5).
References
- Lippa, A; Czobor, P; Stark, J; Beer, B; Kostakis, E; Gravielle, M; Bandyopadhyay, S; Russek, S. J.; Gibbs, T. T.; Farb, D. H.; Skolnick, P (2005). "Selective anxiolysis produced by ocinaplon, a GABAA receptor modulator". Proceedings of the National Academy of Sciences. 102 (20): 7380–7385. doi:10.1073/pnas.0502579102. PMC 1129138. PMID 15870187.
- Slee, April; Nazareth, Irwin; Bondaronek, Paulina; Liu, Yifeng; Cheng, Zhihang; Freemantle, Nick (February 2019). "Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis". The Lancet. 393 (10173): 768–777. doi:10.1016/S0140-6736(18)31793-8. PMID 30712879.
- Mirza, N. R.; Rodgers, R. J.; Mathiasen, L. S. (2006). "Comparative cue generalization profiles of L-838, 417, SL651498, zolpidem, CL218,872, ocinaplon, bretazenil, zopiclone, and various benzodiazepines in chlordiazepoxide and zolpidem drug discrimination". Journal of Pharmacology and Experimental Therapeutics. 316 (3): 1291–9. doi:10.1124/jpet.105.094003. PMID 16339395.
- Atack, J. R. (2005). "The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics". Expert Opinion on Investigational Drugs. 14 (5): 601–18. doi:10.1517/13543784.14.5.601. PMID 15926867.
- http://www.prnewswire.com/news-releases/dov-pharmaceutical-inc-places-ocinaplon-phase-iii-clinical-trial-on-hold-55011422.html
- Baumann, Marcus; Baxendale, Ian R (2013). "An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles". Beilstein Journal of Organic Chemistry. 9: 2265–319. doi:10.3762/bjoc.9.265. PMC 3817479. PMID 24204439.
- ARKIVOC 2010 (ii) 267-282
- "A-Amino Acetals: 2,2-Diethoxy-2-(4-Pyridyl)Ethylamine". Organic Syntheses. 64: 19. 1986. doi:10.15227/orgsyn.064.0019.
- U.S. Patent 4,900,836
- CA 1243029