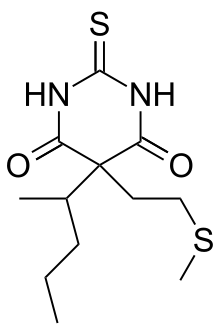
Methitural
Methitural (INN; Neraval, Thiogenal), or methitural sodium, also known as methioturiate, is a barbiturate derivative which was marketed in the 1950s in Europe (in Germany and Italy) as an ultra-short-acting intravenous anesthetic.[1][2][3]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C12H20N2O2S2 |
| Molar mass | 288.429 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Synthesis
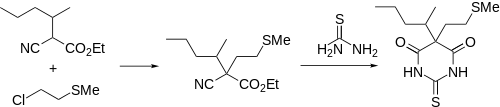
Methitural synthesis: Zima, Von Werder, U.S. Patent 2,802,827 (1957 to E. Merck).
A somewhat more complex side chain is incorporated by alkylation of the carbanion of the substituted cyanoacetate (1) with 2-chloroethylmethyl sulfide (2). Condensation of the resulting cyanoester (3) with thiourea followed by hydrolysis of the resulting imine affords methitural.
See also
References
- F.. Macdonald (1997). Dictionary of Pharmacological Agents. CRC Press. p. 1300. ISBN 978-0-412-46630-4. Retrieved 19 May 2012.
- HOUDE J, HUDON F, JACQUES A (January 1957). "Neraval (methitural sodium) (sch. 3132)". Canadian Anaesthetists' Society Journal. 4 (1): 43–6. doi:10.1007/bf03009193. PMID 13396640.
- IRWIN S, STAGG RD, DUNBAR E, GOVIER WM (March 1956). "Methitural, a new intravenous anesthetic: comparison with thiopental in the cat, dog and monkey". The Journal of Pharmacology and Experimental Therapeutics. 116 (3): 317–25. PMID 13307393.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.