Tiagabine
Tiagabine (trade name Gabitril) is an anticonvulsant medication produced by Cephalon that is used in the treatment of epilepsy. The drug is also used off-label in the treatment of anxiety disorders and panic disorder.
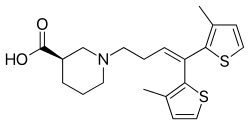 | |
| Clinical data | |
|---|---|
| Pronunciation | /taɪˈæɡəbiːn/ |
| Trade names | Gabitril |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698014 |
| Pregnancy category | |
| Routes of administration | Oral (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90–95%[1] |
| Protein binding | 96%[1] |
| Metabolism | Hepatic (CYP450 system,[1] primarily CYP3A)[2] |
| Onset of action | Tmax = 45 min[2] |
| Elimination half-life | 5–8 hours[3] |
| Excretion | Fecal (63%) and renal (25%)[2] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H25NO2S2 |
| Molar mass | 375.55 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Medical uses
Tiagabine is approved by U.S. Food and Drug Administration (FDA) as an adjunctive treatment for partial seizures in individuals of age 12 and up. It may also be prescribed off-label by physicians to treat anxiety disorders and panic disorder as well as neuropathic pain (including fibromyalgia). For anxiety and neuropathic pain, tiagabine is used primarily to augment other treatments. Tiagabine may be used alongside selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, or benzodiazepines for anxiety, or antidepressants, gabapentin, other anticonvulsants, or opioids for neuropathic pain.[4]
Side effects
The most common side effect of tiagabine is dizziness.[5] Other side effects that have been observed with a rate of statistical significance relative to placebo include asthenia, somnolence, nervousness, memory impairment, tremor, headache, diarrhea, and depression.[5][6] Adverse effects such as confusion, aphasia (difficulty speaking clearly)/stuttering, and paresthesia (a tingling sensation in the body's extremities, particularly the hands and fingers) may occur at higher dosages of the drug (e.g., over 8 mg/day).[5] Tiagabine may induce seizures in those without epilepsy, particularly if they are taking another drug which lowers the seizure threshold.[4] There may be an increased risk of psychosis with tiagabine treatment, although data is mixed and inconclusive.[1][7] Tiagabine can also reportedly interfere with visual color perception.[1]
Overdose
Tiagabine overdose can produce neurological symptoms such as lethargy, single or multiple seizures, status epilepticus, coma, confusion, agitation, tremors, dizziness, dystonias/abnormal posturing, and hallucinations, as well as respiratory depression, tachycardia, hypertension, and hypotension.[8] Overdose may be fatal especially if the victim presents with severe respiratory depression and/or unresponsiveness.[8]
Pharmacology
Tiagabine increases the level of γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system, by blocking the GABA transporter 1 (GAT-1), and hence is classified as a GABA reuptake inhibitor (GRI).[3][9]
History
Tiagabine was discovered at Novo Nordisk in Denmark in 1988 by a team of medicinal chemists and pharmacologists under the general direction of Claus Bræstrup.[10] The drug was co-developed with Abbott Laboratories, in a 40/60 cost sharing deal, with Abbott paying a premium for licensing the IP from the Danish company.
Abbott did initially embrace the drug enthusiastically after its U.S. launch in 1998, and provided further clinical studies with the goal of gaining FDA approval for monotherapy in epilepsy. However, the senior management at Abbott drew back after realizing that the original deal with Novo would limit the company's financial gain from a monotherapy approval. After a period of co-promotion, Cephalon licensed tiagabine from Abbott/Novo and now is the exclusive producer.
U.S. patents on tiagabine listed in the Orange Book expired in April 2016.[11]
References
- Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 562–. ISBN 978-1-60913-345-0.
- "Gabitril (tiagabine hydrochloride) Tablets. U.S. Full Prescribing Information" (PDF). Cephalon, Inc. Retrieved 8 April 2016.
- Brodie, Martin J. (1995). "Tiagabine Pharmacology in Profile". Epilepsia. 36 (s6): S7–S9. doi:10.1111/j.1528-1157.1995.tb06015.x. ISSN 0013-9580. PMID 8595791.
- Stahl, S. Stahl's Essential Psychopharmacology: Prescriber's Guide. Cambridge University Press: New York, NY. 2009. pp. 523-526
- Leppik, Ilo E. (1995). "Tiagabine: The Safety Landscape". Epilepsia. 36 (s6): S10–S13. doi:10.1111/j.1528-1157.1995.tb06009.x. ISSN 0013-9580.
- M.J. Eadie; F. Vajda (6 December 2012). Antiepileptic Drugs: Pharmacology and Therapeutics. Springer Science & Business Media. pp. 459–. ISBN 978-3-642-60072-2.
- J. K. Aronson (2009). Meyler's Side Effects of Psychiatric Drugs. Elsevier. pp. 652–. ISBN 978-0-444-53266-4.
- Spiller, Henry A.; Winter, Mark L.; Ryan, Mark; Krenzelok, Edward P.; Anderson, Debra L.; Thompson, Michael; Kumar, Suparna (2009). "Retrospective Evaluation of Tiagabine Overdose". Clinical Toxicology. 43 (7): 855–859. doi:10.1080/15563650500357529. ISSN 1556-3650.
- Pollack MH, Roy-Byrne PP, Van Ameringen M, Snyder H, Brown C, Ondrasik J, Rickels K (November 2005). "The selective GABA reuptake inhibitor tiagabine for the treatment of generalized anxiety disorder: results of a placebo-controlled study". The Journal of Clinical Psychiatry. 66 (11): 1401–8. doi:10.4088/JCP.v66n1109. PMID 16420077.
- Andersen KE, Braestrup C, Grønwald FC, Jørgensen AS, Nielsen EB, Sonnewald U, Sørensen PO, Suzdak PD, Knutsen LJ (1993). "The synthesis of novel GABA uptake inhibitors. 1. Elucidation of the structure-activity studies leading to the choice of (R)-1-[4,4-bis(3-methyl-2-thienyl)-3-butenyl]-3-piperidinecarboxylic acid (tiagabine) as an anticonvulsant drug candidate". J. Med. Chem. 36 (12): 1716–25. doi:10.1021/jm00064a005. PMID 8510100.
- Orange Book index page. Accessed March 22, 2016
- Carlson, Neil R.; Birkett, Melissa A. (2017). Physiology of Behavior (12 ed.). Psychopharmacology: Pearson. p. 103. ISBN 9780134320823.