ROD-188
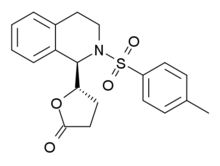
ROD-188 is a sedative drug that was structurally derived from the GABAA antagonist bicuculline by a team at Roche.[1] Unlike bicuculline, ROD-188 acts as an agonist at GABAA receptors, being a positive allosteric modulator acting at a novel binding site distinct from those of benzodiazepines, barbiturates or muscimol, with its strongest effect produced at the α6β2γ2 subtype of the GABAA receptor.[2] ROD-188 is one of a number of related compounds acting at this novel modulatory site, some of which also act at benzodiazepine receptors.[3]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C20H21NO4S |
| Molar mass | 371.449 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
| (verify) | |
See also
References
- US Patent 6649626 N-substituted 1-(lactone) isoquinolones for treating nervous disorders
- Thomet U, Baur R, Razet R, Dodd RH, Furtmüller R, Sieghart W, Sigel E (October 2000). "A novel positive allosteric modulator of the GABA(A) receptor: the action of (+)-ROD188". British Journal of Pharmacology. 131 (4): 843–50. doi:10.1038/sj.bjp.0703558. PMC 1572371. PMID 11030736.
- Sigel E, Baur R, Furtmueller R, Razet R, Dodd RH, Sieghart W (June 2001). "Differential cross talk of ROD compounds with the benzodiazepine binding site". Molecular Pharmacology. 59 (6): 1470–7. doi:10.1124/mol.59.6.1470. PMID 11353808.
| Alcohols |
|
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids |
|
| Imidazoles | |
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators | |
GABA receptor modulators | |||||
|---|---|---|---|---|---|
| Ionotropic |
| ||||
| Metabotropic |
| ||||
| |||||
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.