Pyrazolam
Pyrazolam (SH-I-04)[1] is a benzodiazepine derivative originally developed by a team led by Leo Sternbach at Hoffman-La Roche in the 1970s,[2] and subsequently "rediscovered" and sold as a designer drug starting in 2012.[3][4][5][6][7]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, Sublingual, rectal |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 17 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
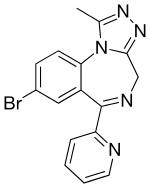
| Formula | C16H12BrN5 |
| Molar mass | 354.211 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Pyrazolam has structural similarities to alprazolam[8] and bromazepam. Unlike other benzodiazepines, pyrazolam does not appear to undergo metabolism, instead being excreted unchanged in the urine.[3] It is most selective for the α2 and α3 subtypes of the GABAA receptor.[9]
Legal Status
United Kingdom
In the UK, pyrazolam has been classified as a Class C drug by section 5 of the May 2017 amendment to The Misuse of Drugs Act 1971 along with several other designer benzodiazepine drugs.[10]
References
- Clayton T, Poe MM, Rallapalli S, Biawat P, Savić MM, Rowlett JK, et al. (2015). "A Review of the Updated Pharmacophore for the Alpha 5 GABA(A) Benzodiazepine Receptor Model". International Journal of Medicinal Chemistry. 2015: 430248. doi:10.1155/2015/430248. PMC 4657098. PMID 26682068.
- US 3954728, "Preparation of triazolo benzodiazepines and novel compounds"
- Moosmann B, Hutter M, Huppertz LM, Ferlaino S, Redlingshöfer L, Auwärter V (July 2013). "Characterization of the designer benzodiazepine pyrazolam and its detectability in human serum and urine". Forensic Toxicology. 31 (2): 263–271. doi:10.1007/s11419-013-0187-4.
- Moosmann B, King LA, Auwärter V (June 2015). "Designer benzodiazepines: A new challenge". World Psychiatry. 14 (2): 248. doi:10.1002/wps.20236. PMC 4471986. PMID 26043347.
- Pettersson Bergstrand M, Helander A, Hansson T, Beck O (April 2017). "Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays". Drug Testing and Analysis. 9 (4): 640–645. doi:10.1002/dta.2003. PMID 27366870.
- Høiseth G, Tuv SS, Karinen R (November 2016). "Blood concentrations of new designer benzodiazepines in forensic cases". Forensic Science International. 268: 35–38. doi:10.1016/j.forsciint.2016.09.006. PMID 27685473.
- Manchester KR, Maskell PD, Waters L (March 2018). "a and plasma protein binding values for benzodiazepines appearing as new psychoactive substances" (PDF). Drug Testing and Analysis. 10 (8): 1258–1269. doi:10.1002/dta.2387. PMID 29582576.
- Hester JB, Rudzik AD, Kamdar BV (November 1971). "6-phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepines which have central nervous system depressant activity". Journal of Medicinal Chemistry. 14 (11): 1078–81. doi:10.1021/jm00293a015. PMID 5165540.
- Hester JB, Von Voigtlander P (November 1979). "6-Aryl-4H-s-triazolo[4,3-a][1,4]benzodiazepines. Influence of 1-substitution on pharmacological activity". Journal of Medicinal Chemistry. 22 (11): 1390–8. doi:10.1021/jm00197a021. PMID 42799.
- "The Misuse of Drugs Act 1971 (Amendment) Order 2017".
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.