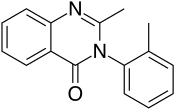
Methaqualone
Methaqualone is a substance once prescribed as a sedative and hypnotic medication, under the brand names Quaalude and Mandrax among others, until commercial production was halted due to widespread abuse, among other things. It is a member of the quinazolinone class.
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /mɛθəˈkweɪloʊn/ |
| Trade names | Bon-Sonnil, Dormogen, Dormutil, Mequin, Mozambin, Pro Dorm, Somnotropon, Torinal, Tuazolona. Methaqualone hydrochloride: Cateudyl, Dormir, Hyptor, Melsed, Melsedin, Mequelon, Methasedil, Nobadorm, Normorest, Noxybel, Optimil, Optinoxan,Pallidan, Parest, Parmilene, Pexaqualone, Renoval, Riporest, Sedalone, Somberol, Somnafac, Somnium,Sopor, Sovelin, Soverin, Sovinal,Toquilone, Toraflon, Tualone, Tuazol. |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 70-80% |
| Elimination half-life | Biphasic (10-40; 20-60 hours) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.710 |
| Chemical and physical data | |
| Formula | C16H14N2O |
| Molar mass | 250.30 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 113 °C (235 °F) |
SMILES
| |
InChI
| |
| (verify) | |
The sedative–hypnotic activity of methaqualone was first noted in the 1950s. In 1962, methaqualone was patented in the United States by Wallace and Tiernan.[1] Its use peaked in the early 1970s for the treatment of insomnia, and as a sedative and muscle relaxant.
Methaqualone became increasingly popular as a recreational drug and club drug in the late 1960s and 1970s, known variously as "ludes" or "sopers" (also "soaps") in the United States and "mandrakes" and "mandies" in the United Kingdom, Australia and New Zealand. The substance was sold both as a free base and as salt (hydrochloride). It is also a common date rape drug.[2]
Medical use
Methaqualone is a sedative that increases the activity of the GABA receptors in the brain and nervous system, similarly to benzodiazepines and barbiturates. When GABA activity is increased, blood pressure drops and the breathing and pulse rates slow, leading to a state of deep relaxation. These properties explain why methaqualone was originally mainly prescribed for insomnia.[3]
Methaqualone was not recommended for use while pregnant and is in pregnancy category D.[4]
Overdose
An overdose can lead to nervous system shutdown, coma and death.[5] Additional effects are delirium, convulsions, hypertonia, hyperreflexia, vomiting, kidney failure, coma, and death through cardiac or respiratory arrest. It resembles barbiturate poisoning, but with increased motor difficulties and a lower incidence of cardiac or respiratory depression. The standard single tablet adult dose of Quaalude brand of methaqualone was 300 mg when made by Lemmon. A dose of 8000 mg is lethal and a dose as little as 2000 mg could induce a coma if taken with an alcoholic beverage.[6]
Pharmacology
Methaqualone peaks in the bloodstream within several hours, with a half-life of 20–60 hours. Regular users build up a physical tolerance, requiring larger doses for the same effect.
History
Methaqualone was first synthesized in India in 1951 by Indra Kishore Kacker and Syed Husain Zaheer, for use as an antimalarial medication.[6][7][8] By 1965, it was the most commonly prescribed sedative in Britain, where it has been sold legally under the names Malsed, Malsedin, and Renoval. In 1965, a methaqualone/antihistamine combination was sold as the sedative drug Mandrax in Europe, by Roussel Laboratories (now part of Sanofi S.A.). In 1972, it was the sixth-bestselling sedative in the US,[9] where it was legal under the brand name Quaalude. Quaalude in the United States was originally manufactured in 1965 by the Fort Washington, Pennsylvania, based pharmaceutical firm William H. Rorer, Inc. The drug name "Quaalude" combined the words "quiet interlude" and shared a stylistic reference to another drug marketed by the firm, Maalox.[10]
In 1978, Rorer sold the rights to manufacture Quaalude to the Lemmon Company of Sellersville, Pennsylvania. At that time, Rorer chairman John Eckman commented on Quaalude's bad reputation stemming from illegal manufacture and use of methaqualone, and illegal sale and use of legally prescribed Quaalude: "Quaalude accounted for less than 2% of our sales, but created 98% of our headaches."[6] Both companies still regarded Quaalude as an excellent sleeping drug. Lemmon, well aware of Quaalude's public image problems, used advertisements in medical journals to urge physicians "not to permit the abuses of illegal users to deprive a legitimate patient of the drug". Lemmon also marketed a small quantity under another name, Mequin, so doctors could prescribe the drug without the negative connotations.[6] The rights to Quaalude were held by the JB Roerig & Company division of Pfizer, before the drug was discontinued in the United States in 1985, mainly due to its psychological addictiveness, widespread abuse, and illegal recreational use.[11]
Society and culture
Brand names
It was sold under the brand name Quaalude and sometimes stylized "Quāālude"[12] in the United States and Mandrax in the United Kingdom and South Africa.
Regulation
Methaqualone was initially placed in Schedule I as defined by the UN Convention of Psychotropic Substances, but was moved to Schedule II in 1979.[13]
In Canada, methaqualone is listed in Schedule III of the Controlled Drugs and Substances Act and requires a prescription, but it is no longer manufactured. Methaqualone is banned in India.[14]
Recreational
Methaqualone became increasingly popular as a recreational drug in the late 1960s and 1970s, known variously as "ludes" or "sopers" (also "soaps") in the United States and "mandrakes" and "mandies" in the UK, Australia and New Zealand.
The drug was more tightly regulated in Britain under the Misuse of Drugs Act 1971 and in the U.S. from 1973. It was withdrawn from many developed markets in the early 1980s. In the United States it was withdrawn in 1982 and made a Schedule I drug in 1984. It has a DEA ACSCN of 2565 and in 2013 the aggregate annual manufacturing quota for the United States was 10 grams. Mention of its possible use in some types of cancer and AIDS has periodically appeared in the literature since the late 1980s; research does not appear to have reached an advanced stage. The DEA has also added the methaqualone analogue mecloqualone (also a result of some incomplete clandestine syntheses) to Schedule I as ACSCN 2572, with zero manufacturing quota.
Gene Haislip, the former head of the Chemical Control Division of the Drug Enforcement Administration (DEA), told the PBS documentary program Frontline, "We beat 'em." By working with governments and manufacturers around the world, the DEA was able to halt production and, Haislip said, "eliminated the problem".[15][16][17] Methaqualone was manufactured in the United States under the name Quaalude by the pharmaceutical firms Rorer and Lemmon with the numbers 714 stamped on the tablet, so people often referred to Quaalude as 714's, "Lemmons", or "Lemmon 7's". Methaqualone was also manufactured in the US under the trade names Sopor and Parest. After the legal manufacture of the drug ended in the United States in 1982, underground laboratories in Mexico continued the illegal manufacture of methaqualone throughout the 1980s, continuing the use of the "714" stamp, until their popularity waned in the early 1990s. Drugs purported to be methaqualone are in a significant majority of cases found to be inert, or contain diphenhydramine or benzodiazepines.
Illicit methaqualone is one of the most commonly used recreational drugs in South Africa. Manufactured in clandestine, often unsanitary labs mainly in India, it comes in tablet form, but is smoked with marijuana; this method of ingestion is known as "white pipe".[18][19] It is also popular elsewhere in Africa and in India.[19]
See also
- Methaqualone in popular culture
- Bill Cosby sexual assault cases
- Drug-facilitated sexual assault
References
- US granted 3135659, Vithal SB, Campanella LA, Hays EE, issued 2 June 1964, assigned to US Filter Wallace and Tiernan Inc
- Brodwin E (2018-04-26). "Bill Cosby has been found guilty - here's what happens if you take the pills he described as 'friends to help you relax'". Business Insider.
- "methaqualone reference". Enotes. Archived from the original on February 23, 2012.
- "Methaqualone in Pregnancy and Breastfeeding". TheDrugSafety.com. Archived from the original on 2012-10-02. Retrieved 15 August 2012.
- "recreational drugs tranquilizers". Drug Library EU. Archived from the original on 2013-03-02.
- Linder L (28 May 1981). "Quaalude manufacturer: Image hurt by street use". Lawrence Journal-World. Associated Press. p. 6. Retrieved 16 August 2013.
Eckman/Fisher
- van Zyl EF (November 2001). "A survey of reported synthesis of methaqualone and some positional and structural isomers". Forensic Science International. 122 (2–3): 142–9. doi:10.1016/S0379-0738(01)00484-4. PMID 11672968.
- Kacker IK, Zaheer SH (1951). "Potential Analgesics. Part I. Synthesis of substituted 4-quinazolones". J. Ind. Chem. Soc. 28: 344–346.
- Foltz RL, Fentiman AF, Foltz RB. GC/MS Assays for Abused Drugs in Body fluids (PDF). National Institutes on Drug Abuse. Washington, D.C: United States Department of Health and Human Services. p. 39.
- "Dividends: Dropping the Last 'Lude". Time. 28 November 1983. Retrieved 16 August 2013.
- Silverstein S. "Quaaludes Again". Captain Wayne's Mad Music.com.
- Rile K (1983). Winter Music (First ed.). Boston and Toronto: Little, Brown and Company. pp. 41, 59. ISBN 978-0-316-74657-1.
- Sandouk L. "green-lists". www.incb.org. Archived from the original on 2017-09-18. Retrieved 2017-09-06.
- "Drugs banned in India". Central Drugs Standard Control Organization, Dte.GHS, Ministry of Health and Family Welfare, Government of India. Archived from the original on 2015-02-21. Retrieved 2013-09-17.
- "The Meth Epidemic – Haislip discusses parallels to current Methamphetamine epidemic".
- Ferns, Sean, "Lecture: Gene Haislip : The Chemical Connection: A Historical Perspective on Chemical Control" Archived 2014-03-31 at the Wayback Machine, Drug Enforcement Administration Museum Lecture Series, Arlington, Virginia, October 25, 2007
- Piccini, Sara, "Drug Warrior: The DEA's Gene Haislip '60, B.C.L. '63 Battled Worldwide Against the Illegal Drug Trade – and Scored a Rare Victory", William & Mary Alumni Magazine, College of William & Mary, Spring 2010
- "Mandrax". DrugAware. Reality Media. 2003. Retrieved 2009-08-13.
- McCarthy G, Myers B, Siegfried N (April 2005). "Treatment for methaqualone dependence in adults". The Cochrane Database of Systematic Reviews (2): CD004146. doi:10.1002/14651858.CD004146.pub2. PMID 15846700.
External links
| Wikimedia Commons has media related to Methaqualone. |
- Erowid Vault – Methaqualone (Quaaludes)
- 2015 Molecular Pharmacology article on functional properties and mechanism of action of Quaaludes