Eterobarb
Eterobarb (Antilon) is a barbiturate derivative. It has mainly anticonvulsant action with less sedative effects than the closely related compound phenobarbital. It saw reasonable success in clinical trials, but is not in widespread medical use.[1][2]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
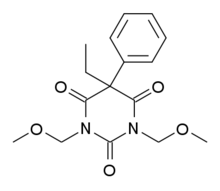
| Formula | C16H20N2O5 |
| Molar mass | 320.340 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Synthesis
Eterobarb can be synthesized by reacting phenobarbital with chloromethyl methyl ether in presence of a base.[3]
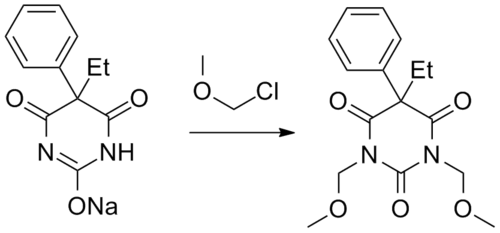
Eterobarb synthesis
References
- Gallagher, BB; Baumel, IP; Woodbury, SG; Dimicco, JA (1975). "Clinical evaluation of eterobarb, a new anticonvulsant drug". Neurology. 25 (5): 399–404. doi:10.1212/wnl.25.5.399. PMID 1094318.
- Smith, DB; Goldstein, SG; Roomet, A (1986). "A comparison of the toxicity effects of the anticonvulsant eterobarb (antilon, DMMP) and phenobarbital in normal human volunteers". Epilepsia. 27 (2): 149–55. doi:10.1111/j.1528-1157.1986.tb03518.x. PMID 3956454.
- Vida, Julius A. (1971). "Anticonvulsants. 1. Alkoxymethyl derivatives of barbiturates and diphenylhydantoin". Journal of Medicinal Chemistry. 14 (3): 187–189. doi:10.1021/jm00285a002.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.