Esketamine
Esketamine, sold under the brand names Ketanest and Spravato, among others,[2][3] is a medication used as a general anesthetic and for treatment-resistant depression.[4][1] Esketamine is used as a nasal spray or by injection into a vein.[4][1]
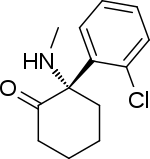 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ketanest, Ketanest S, Spravato, others |
| Other names | Esketamine hydrochloride; (S)-Ketamine; S(+)-Ketamine; JNJ-54135419 |
| AHFS/Drugs.com | International Drug Names |
| License data |
|
| Addiction liability | Low–moderate |
| Routes of administration | Intranasal; Intravenous infusion[1] |
| Drug class | NMDA receptor antagonists; Antidepressants; General anesthetics; Dissociative hallucinogens; Analgesics |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.242.065 |
| Chemical and physical data | |
| Formula | C13H16ClNO |
| Molar mass | 237.725 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Esketamine acts primarily as a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist.[1][5] It also acts to some extent as a dopamine reuptake inhibitor but, unlike ketamine, does not interact with the sigma receptors.[1] The compound is the S(+) enantiomer of ketamine, which is an anesthetic and dissociative similarly.[1] It is unknown whether its antidepressant action is superior, inferior or equal to racemic ketamine and its opposite enantiomer, arketamine, which are both being investigated for the treatment of depression.
Esketamine was introduced for medical use in 1997.[1] In 2019, it was approved for use with other antidepressants, for the treatment of depression in adults in the United States.[6] The cost of the nasal spray as of 2019 will be US$4,700 to $6,800 for the first month.[7]
Medical uses
Anesthesia
Esketamine is a general anesthetic and is used for similar indications as ketamine.[1] Such uses include induction of anesthesia in high-risk patients such as those with hemorrhagic shock, anaphylactic shock, septic shock, severe bronchospasm, severe hepatic insufficiency, cardiac tamponade, and constrictive pericarditis; anesthesia in caesarian section; use of multiple anesthetics in burns; and as a supplement to regional anesthesia with incomplete nerve blocks.[1]
Depression
Similarly to ketamine, esketamine appears to be a rapid-acting antidepressant.[5][8] It received a breakthrough designation from the FDA for treatment-resistant depression (TRD) in 2013 and major depressive disorder (MDD) with accompanying suicidal ideation in 2016.[9][8] The drug was studied specifically for use in combination with an oral antidepressant in people with TRD who had been unresponsive to treatment;[9][5][8] six phase III clinical trials for this indication were conducted in 2017.[9][5][8] It is available as a nasal spray.[9][5][8]
In February 2019, an outside panel of experts recommended that the FDA approve the nasal spray version of esketamine,[10] provided that it be administered in a clinical setting, with patients remaining on site for at least two hours after administration. The reasoning for this requirement is that drug trial participants temporarily experienced sedation, visual disturbances, trouble speaking, confusion, numbness, and feelings of dizziness/faintness during the period immediately after administration.[11]
Side effects
Most common side effects when used in those with treatment resistant depression include dissociation, dizziness, nausea, sleepiness, anxiety, and increased blood pressure.[12]
Pharmacology
Esketamine is approximately twice as potent as an anesthetic as racemic ketamine.[13] It is eliminated from the human body more quickly than arketamine (R(–)-ketamine) or racemic ketamine, although arketamine slows its elimination.[14]
A number of studies have suggested that esketamine has a more medically useful pharmacological action than arketamine or racemic ketamine. However, in mice found that the rapid antidepressant effect of arketamine was greater and lasted longer than that of esketamine.[15] As such, as an antidepressant, the contrary has been stated ("R ketamine appears to be a potent and safe antidepressant relative to S ketamine",[16] "(2R,6R)-HNK (hydroxynorketamine), a major metabolite of (R)-ketamine",[17] "R-ketamine as a longer-lasting antidepressant compared with rapastinel").[18]
Esketamine inhibits dopamine transporters eight times more than arketamine.[19] This increases dopamine activity in the brain. At doses causing the same intensity of effects, esketamine is generally considered to be more pleasant by patients.[20][21] Patients also generally recover mental function more quickly after being treated with pure esketamine, which may be a result of the fact that it is cleared from their system more quickly.[13][22] This is however in contradiction with R-ketamine being devoid of psychotomimetic side effects.[23]
Unlike arketamine, esketamine does not bind significantly to sigma receptors. Esketamine increases glucose metabolism in frontal cortex, while arketamine decreases glucose metabolism in the brain. This difference may be responsible for the fact that esketamine generally has a more dissociative or hallucinogenic effect while arketamine is reportedly more relaxing.[22] However, another study found no difference between racemic and (S)-ketamine on the patient's level of vigilance.[20] Interpretation of this finding is complicated by the fact that racemic ketamine comprises 50% (S)-ketamine.
History
Esketamine was introduced for medical use as an anesthetic in Germany in 1997, and was subsequently marketed in other countries.[1][24] In addition to its anesthetic effects, the medication showed properties of being a rapid-acting antidepressant, and was subsequently investigated for use as such.[5][9] In November 2017, it completed phase III clinical trials for treatment-resistant depression in the United States.[5][9] Johnson & Johnson filed a Food and Drug Administration (FDA) New Drug Application (NDA) for approval on September 4, 2018;[25] the application was endorsed by an FDA advisory panel on February 12, 2019, and on March 5, 2019, the FDA approved esketamine, in conjunction with an oral antidepressant, for the treatment of depression in adults.[6]
Society and culture
Names
Esketamine is the generic name of the drug and its INN and BAN, while esketamine hydrochloride is its BANM.[24] It is also known as S(+)-ketamine, (S)-ketamine, or (–)-ketamine, as well as by its developmental code name JNJ-54135419.[24][9]
Esketamine is marketed under the brand names Spravato for use as an antidepressant and Ketanest, Ketanest S, Ketanest-S, Keta-S for use as an anesthetic (veterinary), among others.[24]
Availability
Esketamine is marketed as an antidepressant in the United States;[6] and as an anesthetic in Europe, including in Austria, Denmark, Estonia, Finland, Germany, the Netherlands, Norway, Slovenia, Sweden, and Switzerland.[24]
Legality
Esketamine is a Schedule III controlled substance in the United States.[4]
References
- Himmelseher S, Pfenninger E (December 1998). "[The clinical use of S-(+)-ketamine--a determination of its place]". Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie. 33 (12): 764–70. doi:10.1055/s-2007-994851. PMID 9893910.
- "Text search results for esketamine: Martindale: The Complete Drug Reference". MedicinesComplete. London, UK: Pharmaceutical Press. Retrieved 20 August 2017.
- Brayfield A, ed. (9 January 2017). "Ketamine Hydrochloride". MedicinesComplete. London, UK: Pharmaceutical Press. Retrieved 20 August 2017.
- "SPRAVATO™ (esketamine) nasal spray FDA label" (PDF). Food and Drug Administration. 5 March 2019. Retrieved 6 March 2019.
- Rakesh G, Pae CU, Masand PS (August 2017). "Beyond serotonin: newer antidepressants in the future". Expert Review of Neurotherapeutics. 17 (8): 777–790. doi:10.1080/14737175.2017.1341310. PMID 28598698.
- Office of the Commissioner. "Press Announcements - FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor's office or clinic". www.fda.gov. Retrieved 2019-03-06.
- "J&J prices ketamine-like depression treatment at $590-$885 for two..." Reuters. 6 March 2019. Retrieved 7 March 2019.
- Lener MS, Kadriu B, Zarate CA (March 2017). "Ketamine and Beyond: Investigations into the Potential of Glutamatergic Agents to Treat Depression" (PDF). Drugs. 77 (4): 381–401. doi:10.1007/s40265-017-0702-8. PMC 5342919. PMID 28194724.
- "Esketamine - Johnson & Johnson - AdisInsight". Retrieved 7 November 2017.
- Koons C, Edney A (February 12, 2019). "First Big Depression Advance Since Prozac Nears FDA Approval". Bloomberg News. Retrieved February 12, 2019.
- Psychopharmacologic Drugs Advisory Committee (PDAC) and Drug Safety and Risk Management (DSaRM) Advisory Committee (February 12, 2019). "FDA Briefing Document" (PDF). Food and Drug Administration. Retrieved February 12, 2019.
Meeting, February 12, 2019. Agenda Topic: The committees will discuss the efficacy, safety, and risk-benefit profile of New Drug Application (NDA) 211243, esketamine 28 mg single-use nasal spray device, submitted by Janssen Pharmaceutica, for the treatment of treatment-resistant depression.
- "Esketamine nasal spray" (PDF). FDA. Retrieved 21 October 2019.
- Himmelseher S, Pfenninger E (December 1998). "[The clinical use of S-(+)-ketamine--a determination of its place]". Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie (in German). 33 (12): 764–70. doi:10.1055/s-2007-994851. PMID 9893910.
- Ihmsen H, Geisslinger G, Schüttler J (November 2001). "Stereoselective pharmacokinetics of ketamine: R(–)-ketamine inhibits the elimination of S(+)-ketamine". Clinical Pharmacology and Therapeutics. 70 (5): 431–8. doi:10.1067/mcp.2001.119722. PMID 11719729.
- Zhang JC, Li SX, Hashimoto K (January 2014). "R (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine". Pharmacology, Biochemistry, and Behavior. 116: 137–41. doi:10.1016/j.pbb.2013.11.033. PMID 24316345.
- Muller J, Pentyala S, Dilger J, Pentyala S (June 2016). "Ketamine enantiomers in the rapid and sustained antidepressant effects". Therapeutic Advances in Psychopharmacology. 6 (3): 185–92. doi:10.1177/2045125316631267. PMC 4910398. PMID 27354907.
- Hashimoto K (November 2016). "Ketamine's antidepressant action: beyond NMDA receptor inhibition". Expert Opinion on Therapeutic Targets. 20 (11): 1389–1392. doi:10.1080/14728222.2016.1238899. PMID 27646666.
- Yang B, Zhang JC, Han M, Yao W, Yang C, Ren Q, Ma M, Chen QX, Hashimoto K (October 2016). "Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression". Psychopharmacology. 233 (19–20): 3647–57. doi:10.1007/s00213-016-4399-2. PMC 5021744. PMID 27488193.
- Nishimura M, Sato K (October 1999). "Ketamine stereoselectively inhibits rat dopamine transporter". Neuroscience Letters. 274 (2): 131–4. doi:10.1016/s0304-3940(99)00688-6. PMID 10553955.
- Doenicke A, Kugler J, Mayer M, Angster R, Hoffmann P (October 1992). "[Ketamine racemate or S-(+)-ketamine and midazolam. The effect on vigilance, efficacy and subjective findings]". Der Anaesthesist (in German). 41 (10): 610–8. PMID 1443509.
- Pfenninger E, Baier C, Claus S, Hege G (November 1994). "[Psychometric changes as well as analgesic action and cardiovascular adverse effects of ketamine racemate versus s-(+)-ketamine in subanesthetic doses]". Der Anaesthesist (in German). 43 Suppl 2: S68–75. PMID 7840417.
- Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J (February 1997). "Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET)". European Neuropsychopharmacology. 7 (1): 25–38. doi:10.1016/s0924-977x(96)00042-9. PMID 9088882.
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K (September 2015). "R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects". Translational Psychiatry. 5 (9): e632. doi:10.1038/tp.2015.136. PMC 5068814. PMID 26327690.
- "Esketamine". Drugs.com.
- "Janssen Submits Esketamine Nasal Spray New Drug Application to U.S. FDA for Treatment-Resistant Depression". Janssen Pharmaceuticals, Inc.