Quingestanol acetate
Quingestanol acetate, sold under the brand names Demovis and Pilomin among others, is a progestin medication which was used in birth control pills but is no longer marketed.[1] It is taken by mouth.[2][3][4]
 | |
| Clinical data | |
|---|---|
| Trade names | Demovis, Pilomin, others |
| Other names | W-4540; Norethisterone acetate 3-cyclopentyl enol ether; 17α-Ethynyl-19-nortestosterone acetate 3-cyclopentyl enol ether; ENTACP; (17β)-3-(Cyclopentyloxy)-17-ethynylestra-3,5-dien-17-yl acetate |
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin; Progestogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ECHA InfoCard | 100.019.163 |
| Chemical and physical data | |
| Formula | C27H36O3 |
| Molar mass | 408.573 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Quingestanol acetate is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[2][3][4] It has weak androgenic and estrogenic activity and no other important hormonal activity.[2][3][4] The medication is a prodrug of norethisterone in the body, with quingestanol and norethisterone acetate occurring as intermediates.[5][6]
Quingestanol acetate was patented in 1963 and was introduced for medical use in 1972.[7][8] It was marketed in Italy.[8]
Medical uses
Quingestanol acetate was used as an oral, once-a-month, or postcoital hormonal contraceptive.[2][3][4]
Side effects
Pharmacology
Quingestanol acetate is a progestogen, and also has weak androgenic and estrogenic activity.[2][3][4] It is a prodrug of norethisterone, with both quingestanol and norethisterone acetate serving as intermediates in the transformation.[5][6] Unlike penmesterol (methyltestosterone 3-cyclopentyl enol ether) and quinestrol (ethinylestradiol 3-cyclopentyl ether), quingestanol acetate is not stored in fat and does not have a prolonged duration of action.[2]
Chemistry
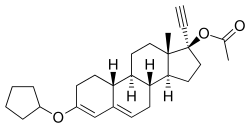
Quingestanol acetate, also known as norethisterone 17β-acetate 3-cyclopentyl enol ether or as 17α-ethynyl-19-nortestosterone 17β-acetate 3-cyclopentyl enol ether (ENTACP), as well as 3-(cyclopentyloxy)-17α-ethynylestra-3,5-dien-17β-yl acetate, is a synthetic estrane steroid and a derivative of testosterone.[1] It is specifically a derivative of 19-nortestosterone and 17α-ethynyltestosterone, or of norethisterone (17α-ethynyl-19-nortestosterone), in which a cyclopentyl enol ether group has been attached at the C3 position and an acetate ester has been attached at the C17β position.[1] Quingestanol acetate is the C17β acetate ester of quingestanol (norethisterone 3-cyclopentyl enol ether).[1]
Society and culture
References
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 1058–. ISBN 978-1-4757-2085-3.
- Giannina T, Steinetz BG, Rassaert CL, McDougall EA, Meli A (July 1969). "Biological profile of quingestanol acetate". Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.). 131 (3): 781–9. doi:10.3181/00379727-131-33977. PMID 5815452.
- Mischler TW, Rubio B, Larranaga A, Guiloff E, Moggia AV (March 1974). "Further experience with quingestanol acetate as a postcoital oral contraceptive". Contraception. 9 (3): 221–5. doi:10.1016/0010-7824(74)90013-4. PMID 4613534.
- Donde UM, Virkar KD (June 1975). "Biochemical studies with once-a-month contraceptive pill containing quinestrol-quingestanol acetate". Contraception. 11 (6): 681–8. doi:10.1016/0010-7824(75)90065-7. PMID 1137940.
- Raynaud JP, Ojasoo T (1986). "The design and use of sex-steroid antagonists". J. Steroid Biochem. 25 (5B): 811–33. doi:10.1016/0022-4731(86)90313-4. PMID 3543501.
Similar androgenic potential is inherent to norethisterone and its prodrugs (norethisterone acetate, ethynodiol diacetate, lynestrenol, norethynodrel, quingestanol).
- Di Carlo FJ, Loo JC, Aceto T, Zuleski FR, Barr WH (1974). "Quingestanol acetate metabolism in women". Pharmacology. 11 (5): 287–303. doi:10.1159/000136501. PMID 4853997.
- Lara Marks (2010). Sexual Chemistry: A History of the Contraceptive Pill. Yale University Press. pp. 73–. ISBN 978-0-300-16791-7.
- Population Reports: Oral contraceptives. Department of Medical and Public Affairs, George Washington Univ. Medical Center. 1975. p. A-64.
- Janne S. Kowalski (1 August 1988). Drug companies & products world guide. Sittig & Noyes. p. 388.