Methenmadinone acetate
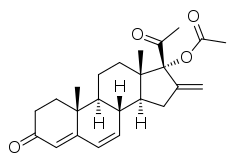
Methenmadinone acetate (MMA), also known as methylenedehydroacetoxyprogesterone (MDAP) and sold under the brand names Superlutin and Antigest, is a progestin medication which was developed in Czechoslovakia in the 1960s.[1][2][3][4][5][6][7][8][9][10][11][12][13] It is the C17α acetate ester of methenmadinone.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Superlutin, Antigest |
| Other names | Superlutin; Superlutine; MMA; Methylenedehydroacetoxyprogesterone; MDAP; 17α-Hydroxy-16-methylene-δ6-progesterone 17α-acetate; 17α-Acetoxy-16-methylenepregna-4,6-diene-3,20-dione |
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin; Progestogen ester |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C24H30O4 |
| Molar mass | 382.5 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
MMA given orally shows about 13-fold the progestogenic activity of parenteral progesterone in animal bioassays.[14]
Analogues of methenmadinone acetate include methenmadinone caproate (MMC), which was studied in combination with estradiol valerate as a combined injectable contraceptive (tentative brand name Lutofollin);[15][16][17] chlormethenmadinone acetate (chlorsuperlutin; SCH-12600; 6-chloro-MMA),[18] which has been used in combination with mestranol in birth control pills (brand names Biogest, Sterolibrin, Antigest B)[19][20] and in veterinary medicine (brand name Agelin);[21] bromethenmadinone acetate (bromsuperlutin; 6-bromo-MMA), which was assessed but was never marketed;[20][22] and melengestrol acetate (methylsuperlutin; 6-methyl-MMA), which is used in veterinary medicine.[6]
See also
References
- G.W.A Milne (1 November 2017). Ashgate Handbook of Endocrine Agents and Steroids. Taylor & Francis. pp. 158–. ISBN 978-1-351-74347-1.
- J. Horsky; J. Presl (6 December 2012). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 93, 318. ISBN 978-94-009-8195-9.
- Cynthia A Challener (23 October 2017). Chiral Drugs. Taylor & Francis. pp. 1127–. ISBN 978-1-351-80804-0.
- Horský, Jan; Presl, Jiří (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". Ovarian Function and its Disorders. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8197-3.
- J.P. Lavery; J.S. Sanfilippo (6 December 2012). Pediatric and Adolescent Obstetrics and Gynecology. Springer Science & Business Media. pp. 236–. ISBN 978-1-4612-5064-7.
- R. G. Denkewalter; M. Tishler; G. Ehrhart; J. H. Biel, B. K. B. Lum, J. Büchi, C. A. Winter, K. Münzel, W. Kunz, E. J. Ariëns, F. Labhardt (8 March 2013). Fortschritte der Arzneimittelforschung / Progress in Drug Research / Progrès des recherches pharmaceutiques. Birkhäuser. pp. 407–. ISBN 978-3-0348-7059-7.CS1 maint: multiple names: authors list (link)
- HONTELA S (September 1964). "[Clinical experience with the new peroral gestagen, 16-methylene-6-dehydro-17-alpha-acetoxyprogesterone (MDAP)]". Zentralbl Gynakol (in German). 86: 1327–32. PMID 14324038.
- Králová A, Stĕrba R, Valová B (June 1965). "[Superlutin--a new Czechoslovak oral gestagen]". Cesk Gynekol (in Czech). 30 (5): 351–5. PMID 5835891.
- Dvorák K, Presl J (November 1986). "[Biological testing of methenmadinone acetate (Superlutin Spofa) in clinical trials]". Cesk Gynekol (in Czech). 51 (9): 714–9. PMID 3539369.
- Drázdil M (December 1966). "[Superlutin]". Cesk Farm (in Czech). 15 (10): 543–4. PMID 5980773.
- Horák C, Sedliak M (February 1966). "[Our experience in the treatment of menstruation disorders with Superlutin]". Cesk Gynekol (in Slovak). 31 (1): 86–8. PMID 5932092.
- Stĕrba R (July 1967). "[3-Methylether-ethinylestradiol (Mestranol) and 6-dehydro-16-methylene-17-alpha-acetoxyprogesterone (Superlutin) in the therapy of functional bleedings]". Zentralbl Gynakol (in German). 89 (28): 1030–1. PMID 5586402.
- Sterba, R. (1969). Several experiences with injected progesterones. Ceskoslovenska Gynekologie, 34(7), 407-409. https://www.popline.org/node/506944
- Čekan, Z.; Šeda, M.; Mikulášková, J.; Syhora, K. (1964). "Steroid derivatives XXXIV. On the progestational activity of 6-dehydro-16-methylene-17α-acetoxyprogesterone". Steroids. 4 (3): 415–421. doi:10.1016/0039-128X(64)90154-0. ISSN 0039-128X.
- Stĕrba R (1976). "[A Czechoslovak injection-contraceptive agent administered once a month]". Zentralbl Gynakol (in German). 98 (3): 158–60. PMID 970015.
- Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- Goldsmith, A., & Toppozada, M. (1983). Long-acting contraception. pp. 94-95 https://www.popline.org/node/423289
- Sterba, R. (1968). New biological application of contraceptive steroids. Endocrinologia Experimentalis, 2(2), 101-110. https://www.popline.org/node/469522
- Melich H (July 1972). "[Biogest]". Cas. Lek. Cesk. (in Czech). 111 (30): 694–5. PMID 5079918.
- Stĕrba R (March 1970). "[Towards a more physiological hormonal contraception]". Zentralbl Gynakol (in German). 92 (10): 303–12. PMID 4096927.
- Bekeová E, Krajnicáková M, Hendrichovský V, Maracek I (November 1995). "[Thyroid and ovarian hormones in ewes treated with gestagens and PMSG in the spring season]". Vet Med (Praha) (in Slovak). 40 (11): 345–52. PMID 8659087.
- Štěrba, R. (1971). "On the Way to a More Physiological Hormonal Contraception". Current Problems in Fertility. pp. 154–158. doi:10.1007/978-1-4615-8651-7_28. ISBN 978-1-4615-8653-1.