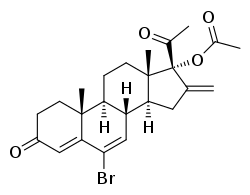
Bromethenmadinone acetate
Bromethenmadinone acetate (BMMA, also known as bromsuperlutin) is a progestin medication which was developed in Czechoslovakia and was described in 1970 but was never marketed.[1][2][3][4][5][6][7][8][9] Analogues of BMMA include chlormethenmadinone acetate, melengestrol acetate, and methenmadinone acetate.
 | |
| Clinical data | |
|---|---|
| Other names | BMMA; Bromsuperlutin; 6-Bromo-16-methylene-17α-hydroxy-Δ6-progesterone acetate; 6-Bromo-16-methylene-17α-acetoxypregna-4,6-diene-3,20-dione |
| Drug class | Progestogen; Progestin; Progestogen ester |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C24H29BrO4 |
| Molar mass | 461.396 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
References
- Stĕrba R (March 1970). "[Towards a more physiological hormonal contraception]". Zentralbl Gynakol (in German). 92 (10): 303–12. PMID 4096927.
- Štěrba, R. (1971). "On the Way to a More Physiological Hormonal Contraception". Current Problems in Fertility. pp. 154–158. doi:10.1007/978-1-4615-8651-7_28. ISBN 978-1-4615-8653-1.
- Cekan Z, Horesovský O (February 1971). "Elimination and metabolism of 6-chloro-17-alpha-hydroxy-16-methylene-4,6-pregnadiene-3,20-dione acetate and its analogues in rats". Acta Endocrinol. 66 (2): 303–16. doi:10.1530/acta.0.0660303. PMID 5107826.
- Shapiro EL, Weber L, Harris H, Miskowicz C, Neri R, Herzog HL (July 1972). "Synthesis and biological activity of 17-esters of 6-dehydro-16-methylene-17 -hydroxyprogesterones". J. Med. Chem. 15 (7): 716–20. doi:10.1021/jm00277a006. PMID 5043870.
- Míčková, R. (1973). "Steroid derivatives. LXXV. The preparation of polyhalogenated derivatives of progestational hormones and the determination of their conformation by circular dichroism spectra". Collection of Czechoslovak Chemical Communications. 38 (8): 2492–2503. doi:10.1135/cccc19732492. ISSN 0010-0765.
- Wolff ME (August 1974). "A quantitative reexamination of structure-activity relationships in the delta6-6-substituted progesterone series". J. Med. Chem. 17 (8): 898–900. doi:10.1021/jm00254a025. PMID 4845383.
- Topliss JG, Shapiro EL (June 1975). "Quantitative structure-activity relationships in the delta6-6-substituted progesterone series. A reappraisal". J. Med. Chem. 18 (6): 621–3. doi:10.1021/jm00240a020. PMID 1151979.
- Wolff ME, Hansch C, Giannini DD, Kollman PA, Duax WL, Baxter J (1975). "Correlation of glucocorticoid and progestational activity with steric, electronic and hydrophobic parameters". J. Steroid Biochem. 6 (3–4): 211–4. doi:10.1016/0022-4731(75)90134-X. PMID 171485.
- Coburn RA, Solo AJ (June 1976). "Quantitative structure-activity relationships among steroids. Investigations of the use of steric parameters". J. Med. Chem. 19 (6): 748–54. doi:10.1021/jm00228a002. PMID 950640.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.