Pharmacokinetics of estradiol
The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.[9][10][11]
 | |
| Clinical data | |
|---|---|
| Routes of administration | • By mouth (tablet) • Sublingual (tablet) • Intranasal (nasal spray) • Transdermal (patch, gel, cream, emulsion, spray) • Vaginal (tablet, cream, suppository, insert, ring) • IM injection (oil solution) • SC injection (aq. soln.) • Subcutaneous implant |
| Drug class | Estrogen; Antigonadotropin |
| Pharmacokinetic data | |
| Bioavailability | Oral: 5% (0.1–12%)[1][2] SL: 10% (in marmosets)[3] IM: 100%[4] |
| Protein binding | ~98%:[1][5] • Albumin: 60% • SHBG: 38% • Free: 2% |
| Metabolism | Liver (via hydroxylation, sulfation, glucuronidation) |
| Metabolites | Major (90%):[1] • Estrone • Estrone sulfate • Estrone glucuronide • Estradiol glucuronide |
| Elimination half-life | Oral: 13–20 hours[1] Sublingual: 8–18 hours[6] Transdermal (gel): 37 hours[7] IM (as EV): 4–5 days[4] IM (as EC): 8–10 days[8] IV (as E2): 1–2 hours[4] |
| Excretion | Urine: 54%[1] Feces: 6%[1] |
Estradiol is a naturally occurring and bioidentical estrogen, or an agonist of the estrogen receptor, the biological target of estrogens like endogenous estradiol.[9] Due to its estrogenic activity, estradiol has antigonadotropic effects and can inhibit fertility and suppress sex hormone production in both women and men.[12][13] Estradiol differs from non-bioidentical estrogens like conjugated estrogens and ethinylestradiol in various ways, with implications for tolerability and safety.[9]
Estradiol can be taken by mouth, held under the tongue, as a gel or patch that is applied to the skin, in through the vagina, by injection into muscle or fat, or through the use of an implant that is placed into fat, among other routes.[9]
Routes of administration
Estradiol can be taken by a variety of different routes of administration.[9] These include oral, buccal, sublingual, intranasal, transdermal (gels, creams, patches), vaginal (tablets, creams, rings, suppositories), rectal, by intramuscular or subcutaneous injection (in oil or aqueous), and as a subcutaneous implant.[9] The pharmacokinetics of estradiol, including its bioavailability, metabolism, biological half-life, and other parameters, differ by route of administration.[9] Likewise, the potency of estradiol, and its local effects in certain tissues, most importantly the liver, differ by route of administration as well.[9] In particular, the oral route is subject to a high first-pass effect, which results in high levels of estradiol and consequent estrogenic effects in the liver and low potency due to first-pass hepatic and intestinal metabolism into metabolites like estrone and estrogen conjugates.[9] Conversely, this is not the case for parenteral (non-oral) routes, which bypass the intestines and liver.[9]
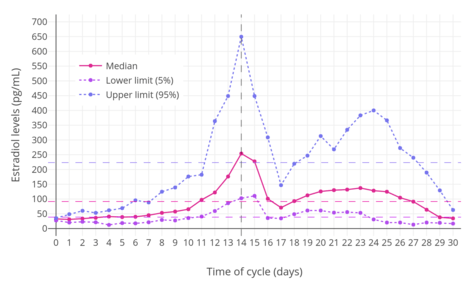
Different estradiol routes and dosages can achieve widely varying circulating estradiol levels (see the table below).[9] For purposes of comparison with normal physiological circumstances, menstrual cycle circulating levels of estradiol in premenopausal women are 40 pg/mL in the early follicular phase, 250 pg/mL at the middle of the cycle, and 100 pg/mL during the mid-luteal phase.[15] Mean integrated levels of circulating estradiol in premenopausal women across the whole menstrual cycle are in the range of 80 to 150 pg/mL, according to some sources.[16][17][18] In postmenopausal women, circulating levels of estradiol are below 15 pg/mL.[9][15] During normal human pregnancy, estrogen production increases progressively and extremely high estrogen levels are attained.[19] Estradiol levels range from 1,000 to 40,000 pg/mL across pregnancy,[14] are on average 25,000 pg/mL at term, and reach levels as high as 75,000 pg/mL in some women.[20]
| Route | Ingredient | Form | Dose | Major brand names |
|---|---|---|---|---|
| Oral | Estradiol | Tablet | 0.1, 0.2, 0.5, 1, 2, or 4 mg per tablet | Estrace, Ovocyclin |
| Estradiol acetatea | Tablet | 0.45, 0.9, or 1.8 mg per tablet | Femtrace | |
| Estradiol valerate | Tablet | 0.5, 1, 2, or 4 mg per tablet | Progynova | |
| Sublingual | Estradiola | Tablet | 0.125, 0.25, or 1 mg per tablet | Diogynets, Estradiol Membrettes |
| Intranasal | Estradiola | Nasal spray | 150 µg per spray (60 sprays per bottle) | Aerodiol |
| Transdermal | Estradiol | Patch | 14, 25, 37.5, 50, 60, 75, or 100 µg E2 per day for 3–4 or 7 days | Climara, Estraderm, Vivelle |
| Gel dispenser | 0.06% (0.87 or 1.25 g gel or 0.52 or 0.75 mg E2 per activation) | Elestrin, EstroGel | ||
| Gel packet | 0.1% (0.25, 0.5, or 1 g gel or 2.5, 5, or 10 mg E2 per packet) | DiviGel, Sandrena | ||
| Emulsion | 0.14% (1.74 g emulsion or 4.35 mg E2 per pouch; 50 µg/day E2) | Estrasorb | ||
| Spray | 1.53 mg per spray | Evamist | ||
| Vaginal | Estradiol | Tablet | 10 or 25 µg per tablet | Vagifem |
| Cream | 0.01% (0.1 mg E2 per 1 g cream) | Estrace | ||
| Suppositorya | 4 or 40 μg per suppository | Ovocyclin | ||
| Insert | 4 or 10 µg per insert (daily for 2 weeks then twice weekly) | Imvexxy | ||
| Ring | 2 mg per ring (7.5 µg/day E2 for 3 months) | Estring | ||
| Estradiol acetate | Ring | 12.4 or 24.8 mg per ring (50 or 100 µg/day E2 for 3 months) | Femring | |
| Injection (IM or SC) | Estradiol | Microspheres | 1 mg/mL | Juvenum E |
| Estradiol benzoate | Oil solution | 0.167, 0.2, 0.333, 1, 1.67, 2, 5, 10, 20, or 25 mg/mL | Progynon-B | |
| Aqueous suspensiona | 5 mg/mL | Agofollin Depot | ||
| Estradiol cypionate | Oil solution | 1, 3, or 5 mg/mL | Depo-Estradiol | |
| Aqueous suspension | 5 mg/0.5 mL (available only with a progestin) | Cyclofem, Lunelle | ||
| Estradiol dipropionatea | Oil solution | 0.1, 0.2, 0.5, 1, 2.5, or 5 mg/mL | Di-Ovocylin, Progynon-DP | |
| Estradiol enantate | Oil solution | 5 or 10 mg/mL (available only with a progestin) | Perlutal, Topasel | |
| Estradiol undecylatea | Oil solution | 100 mg/mL | Delestrec, Progynon Depot 100 | |
| Estradiol valerate | Oil solution | 5, 10, 20, or 40 mg/mL | Delestrogen, Progynon Depot | |
| Polyestradiol phosphatea | Aqueous solution | 40 or 80 mg per vial/ampoule | Estradurin | |
| Implant | Estradiola | Pellet | 20, 25, 50, or 100 mg per pellet (usually every 6 months) | Estradiol Implants, Meno-Implant |
| Abbreviations: E2 = Estradiol. Footnotes: a = Discontinued or mostly discontinued. Notes: (1): This table mostly does not include combination products, for instance estradiol formulated in combination with a progestogen or androgen. (2): This table does not include compounded estradiol products; only approved pharmaceutical preparations are included. (3): The availability of pharmaceutical estradiol products differs by country (see Estradiol (medication) § Availability). (4): Some of these formulations and doses have been marketed previously but may no longer be available. Sources: See template. | ||||
| Route | Dose | Time | E2 (↑Δ) | E1 (↑Δ) | Ratio | ||||
|---|---|---|---|---|---|---|---|---|---|
| Oral | 1 mg 2 mg 4 mg | 12 hours 3 hours 6 hours | +25 pg/mL +40 pg/mL +50 pg/mL | +150 pg/mL +250 pg/mL +500 pg/mL | 0.15 0.16 0.10 | ||||
| Sublingual | 1 mg 0.5 mg 0.5 mg 0.5 mg | 1 hour 1 hour 1 hour 1 hour | +450 pg/mL +250 pg/mL +750 pg/mL +75 pg/mL | +160 pg/mL +85 pg/mL +250 pg/mL +24 pg/mL | 3 3 3 3 | ||||
| Intranasal | 1 mg | 1 hour | +110 pg/mL | +110 pg/mL | 1.0 | ||||
| Topical (gel) | 3 mg 3 mg/day 3 mg/2 days | 5 hours 12–20 hours 12 hours 36 hours | +70 pg/mL +45–279 pg/mL +300–1310 pg/mL +20–179 pg/mL | +50 pg/mL +31–230 pg/mL +24–110 pg/mL +120–500 pg/mL | 0.4 1 1 1 | ||||
| Vaginal (cream) | 0.5 mg 1.0 mg | 3 hours 3 hours | +830 pg/mL +800 pg/mL | +150 pg/mL +150 pg/mL | 5.0 5.0 | ||||
| Rectal | 1 mg | 3 hours | +620 pg/mL | +120 pg/mL | 5.0 | ||||
| Intramuscular (esters in oil) | 5 mg EB 5 mg EV 5 mg EC 100 mg EU 320 mg PEP | 1.8, 2.4 daysa 2.2, 2.7 daysa 3.9, 5.1 daysa 1 day 16, 25 daysa | 940 pg/mLb 667 pg/mLb 338 pg/mLb 500 pg/mLb 270 pg/mLb | 343 pg/mLb 324 pg/mLb 145 pg/mLb ND 1000 pg/mLb | 2.7 2.1 2.3 ND 0.27 | ||||
| Intravenous | 0.3 mg | 5 minutes | 8321 pg/mLb | 960 pg/mLb | 8.7 | ||||
| Footnotes: a = Tmax for E2, E1 levels. b = Actual levels (not change). Sources: See template. | |||||||||
| Group | E2 (prod) | E2 (levels) | E1 (levels) | Ratio |
|---|---|---|---|---|
| Pubertal girlsa Tanner stage I (childhood) Tanner stage II (ages 8–12) Tanner stage III (ages 10–13) Tanner stage IV (ages 11–14) Tanner stage V (ages 12–15) Follicular (days 1–14) Luteal (days 15–28) | ? ? ? ? ? ? | 9 (<9–20) pg/mL 15 (<9–30) pg/mL 27 (<9–60) pg/mL 55 (16–85) pg/mL 50 (30–100) pg/mL 130 (70–300) pg/mL | 13 (<9–23) pg/mL 18 (10–37) pg/mL 26 (17–58) pg/mL 36 (23–69) pg/mL 44 (30–89) pg/mL 75 (39–160) pg/mL | ? ? ? ? ? ? |
| Prepubertal boys | ? | 2–8 pg/mL | ? | ? |
| Premenopausal women Early follicular phase (days 1–4) Mid follicular phase (days 5–9) Late follicular phase (days 10–14) Luteal phase (days 15–28) Oral contraceptive (anovulatory) | 30–100 µg/day 100–160 µg/day 320–640 µg/day 300 µg/day ? | 40–60 pg/mL 60–100 pg/mL 200–400 pg/mL 190 pg/mL 12–50 pg/mL | 40–60 pg/mL ? 170–200 pg/mL 100–150 pg/mL ? | 0.5–1 ? 1–2 1.5 ? |
| Postmenopausal women | 18 µg/day | 5–20 pg/mL | 30–70 pg/mL | 0.3–0.8 |
| Pregnant women First trimester (weeks 1–12) Second trimester (weeks 13–26) Third trimester (weeks 27–40) | ? ? ? | 1,000–5,000 pg/mL 5,000–15,000 pg/mL 10,000–40,000 pg/mL | ? ? ? | ? ? ? |
| Mena | 20–60 µg/day | 27 (20–55) pg/mL | 20–90 pg/mL | 0.4–0.6 |
| Footnotes: a = Format is "Mean value (range)". Sources: See template. | ||||
Oral administration
Absorption and bioavailability
The oral bioavailability of estradiol is very low, and the hormone is micronized and/or conjugated with an ester, as in estradiol valerate or estradiol acetate, to improve its bioavailability such that it is more clinically useful.[2][21][22] This is because estradiol is extensively metabolized during the first pass through the intestines and liver.[3] Micronization decreases the size of estradiol particles and thereby increases the surface area for absorption.[23] This, in turn, increases the rate of absorption of estradiol and improves its metabolic stability.[3] Oral micronized estradiol consists of greater than 80% of substance particles micronized to a size smaller than 20 μM in diameter.[24][25] All oral estradiol tablets available today are micronized.[21] Similarly, all oral estradiol valerate tablets appear to be micronized.[26] Oral non-micronized estradiol and oral micronized estradiol do not seem to have ever been directly compared in a study, but have been assessed independently.[27][25][28][29] They both appear to be potently estrogenic.[27][25][28][29] Micronization of norethisterone acetate has been found to increase its potency by several-fold.[30][31][32][33] In accordance, studies of the amount of oral estradiol necessary for endometrial proliferation in women have reported a total dose of 60 mg for micronized estradiol[34] and 120 to 300 mg or more for non-micronized estradiol.[35][36][37]
Micronized estradiol is rapidly and completely absorbed with oral administration.[3][11] This is true for oral doses of 2 mg and 4 mg, but absorption was found to be incomplete for an oral dose of 8 mg (which showed 76% of the expected bioavailability, based on dose proportionality and area-under-the-curve levels).[3][38] The absolute bioavailability of oral micronized estradiol is approximately 5%, with a possible range of 0.1% to 12%.[1][2] There is high interindividual variability in the levels of estradiol achieved with oral estradiol, which is likely related to the high first-pass effect.[11] This variation has been reported to be in the range of 28 to 127%, or about 4.6-fold maximal difference in levels between individuals, in terms of mean area-under-the-curve levels of estradiol.[11]
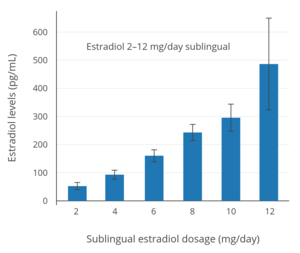
In postmenopausal women, a dosage of 1 mg/day oral micronized estradiol has been found to produce circulating concentrations of 30 to 50 pg/mL estradiol and 150 to 300 pg/mL estrone, while a dosage of 2 mg/day has been found to result in circulating levels of 50 to 180 pg/mL estradiol and 300 to 850 pg/mL estrone.[15] A study of oral micronized estradiol in transgender women found that mean estradiol levels across a dosage range of 1 to 8 mg/day were almost 50 pg/mL at 1 mg/day, almost 100 pg/mL at 4 mg/day, and just above 150 pg/mL at 8 mg/day, with a wide degree of variation.[39] A single 10 mg dose of micronized estradiol or estradiol valerate has been found to produce circulating levels of estradiol of about 250 pg/mL in postmenopausal women.[40] A study that used high- to very high-dose oral micronized estradiol to treat postmenopausal women with ER-positive breast cancer found that mean steady-state estradiol levels in the 6 mg/day group were about 300 pg/mL and in the 30 mg/day group were about 2,400 pg/mL.[41]
| Estrogen | Type | Class | ETD (mg/14 days) | EPD (mg/14 days) | EPD (mg/day) | MSD (mg/14 days) | MSD (mg/day) | TSD (mg/day) |
|---|---|---|---|---|---|---|---|---|
| Estradiol (non-micronized) | Bioidentical | Steroidal | ? | ≥120–300 | ? | ? | ? | ? |
| Estradiol (micronized) | Bioidentical | Steroidal | ? | 60–80 | 4.3 | 14–28 | 1.0–2.0 | >8 |
| Estradiol valerate | Bioidentical | Steroidal | 6–10 | 60–80 | 4.3 | 14–28 | 1.0–2.0 | >8 |
| Estradiol benzoate | Bioidentical | Steroidal | ? | 60–140 | 4.5 | ? | ? | ? |
| Estriol | Bioidentical | Steroidal | 20a | 120–150b | 10.0–10.7b | 28–84 | 1.0–6.0 | ? |
| Estriol succinate | Bioidentical | Steroidal | ? | 140–150b | 10.0–10.7b | 28–84 | 2.0–6.0 | ? |
| Conjugated estrogens | Natural | Steroidal | 5–12 | 60–80 | 4.3 | 8.4–17.5 | 0.625–1.25 | 7.5 |
| Ethinylestradiol | Synthetic | Steroidal | 0.2 | 1.0–2.0 | 0.071–0.11 | 0.28 | 0.02–0.04 | 0.1 |
| Mestranol | Synthetic | Steroidal | 0.3 | 1.5–3.0 | 0.11–0.13 | 0.3–0.5 | 0.025 | ? |
| Quinestrol | Synthetic | Steroidal | 0.3 | 2.0–4.0 | 0.14–0.29 | ? | 0.025–0.05 | ? |
| Methylestradiol | Synthetic | Steroidal | ? | 2.0 | ? | ? | ? | ? |
| Diethylstilbestrol | Synthetic | Nonsteroidal | 2.5 | 20–30 | 1.4–2.1 | ? | 0.5–2.0 | 3 |
| Diethylstilbestrol dipropionate | Synthetic | Nonsteroidal | ? | 15–30 | 1.1–1.4 | ? | ? | ? |
| Dienestrol | Synthetic | Nonsteroidal | ? | 30 | ? | ? | 0.5–4.0 | ? |
| Dienestrol diacetate | Synthetic | Nonsteroidal | 3–5 | 30–60 | 2.9–4.3 | ? | ? | ? |
| Hexestrol | Synthetic | Nonsteroidal | ? | 70–110 | ? | ? | ? | ? |
| Hexestrol diacetate | Synthetic | Nonsteroidal | ? | 45 | ? | ? | ? | ? |
| Chlorotrianisene | Synthetic | Nonsteroidal | ? | >100 | ? | ? | ? | ? |
| Methallenestril | Synthetic | Nonsteroidal | ? | 400 | ? | ? | ? | ? |
| Note: The OID of EE is 0.1 mg/day. Footnotes: a = Very variable, often higher. b = In divided doses, 3x/day; irregular and atypical proliferation. Sources: See template. | ||||||||
| Estrogen | Type | HF | VE | UCa | FSH | LH | HDL-C | SHBG | CBG | AGT | Liver |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol | Bioidentical | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Estrone | Bioidentical | ? | ? | ? | 0.3 | 0.3 | ? | ? | ? | ? | ? |
| Estriol | Bioidentical | 0.3 | 0.3 | 0.1 | 0.3 | 0.3 | 0.2 | ? | ? | ? | 0.67 |
| Estrone sulfate | Bioidentical | ? | 0.9 | 0.9 | 0.8–0.9 | 0.9 | 0.5 | 0.9 | 0.5–0.7 | 1.4–1.5 | 0.56–1.7 |
| Conjugated estrogens | Natural | 1.2 | 1.5 | 2.0 | 1.1–1.3 | 1.0 | 1.5 | 3.0–3.2 | 1.3–1.5 | 5.0 | 1.3–4.5 |
| Equilin sulfate | Natural | ? | ? | 1.0 | ? | ? | 6.0 | 7.5 | 6.0 | 7.5 | ? |
| Ethinylestradiol | Synthetic | 120 | 150 | 400 | 60–150 | 100 | 400 | 500–600 | 500–600 | 350 | 2.9–5.0 |
| Diethylstilbestrol | Synthetic | ? | ? | ? | 2.9–3.4 | ? | ? | 26–28 | 25–37 | 20 | 5.7–7.5 |
| Notes: Values are ratios, with estradiol as standard (i.e., 1.0). Abbreviations: HF = Clinical relief of hot flashes. VE = Increased proliferation of vaginal epithelium. UCa = Decrease in UCa. FSH = Suppression of FSH levels. LH = Suppression of LH levels. HDL-C, SHBG, CBG, and AGT = Increase in the serum levels of these liver proteins. Liver = Ratio of liver estrogenic effects to general/systemic estrogenic effects (specifically hot flashes relief and gonadotropin suppression). Type: Bioidentical = Identical to those found in humans. Natural = Naturally occurring but not identical to those found in humans (e.g., estrogens of other species). Synthetic = Man-made, does not occur naturally in animals or in the environment. Sources: See template. | |||||||||||
Metabolism and elimination
When taken orally, about 95% of a dose of estradiol is metabolized in the intestines and liver into estrone and estrogen conjugates such as estrone sulfate, estrone glucuronide, and estradiol sulfate, among others, prior to entering the circulation.[3][42][43][44] As a result, circulating estrone and estrogen conjugate levels are markedly elevated, in a highly unphysiological manner, with oral estradiol.[42][45] Whereas the ratio of circulating estradiol to estrone is about 1:1 in premenopausal women and with transdermal estradiol, oral estradiol produces a ratio of about 1:5 on average and as high as 1:20 in some women.[1][9][46][38] In addition, whereas levels of estradiol with menopausal replacement dosages of oral estradiol are in the range of the follicular phase of the normal menstrual cycle, levels of estrone resemble those during the first trimester of pregnancy.[47][48] Moreover, whereas normal physiological estrone sulfate levels are 10 to 25 times higher than those of estradiol and estrone in premenopausal women,[49] levels of estrone sulfate with oral estradiol are an additional 8 to 20 times higher than normal premenopausal or postmenopausal estrone sulfate levels.[45][50][51] One study found that estrone sulfate levels were 200-fold higher than estradiol levels with 2 mg/day oral micronized estradiol or oral estradiol valerate, and estrone sulfate levels can reach up to nearly 1,000-fold higher concentrations than estradiol in some cases.[9][11] In contrast to oral estradiol, due to the lack of the first pass, an excess in estrone and estrogen conjugate levels does not occur with transdermal estradiol or other parenteral estradiol routes.[42][45]
The transformation of estradiol into estrone and estrogen conjugates is reversible, and these metabolites hence can be converted back into estradiol.[9] About 15% of orally administered estradiol is transformed into estrone and 65% into estrone sulfate.[11] About 5% of estrone and 1.4% of estrone sulfate can be converted back into estradiol.[11] An additional 21% of estrone sulfate can be converted into estrone, whereas the transformation of estrone into estrone sulfate is approximately 54%.[11] The interconversion between estradiol and estrone is mediated by 17β-hydroxysteroid dehydrogenases (17β-HSDs),[11] whereas the conversion of estrone into estrone sulfate is mediated by estrogen sulfotransferases (ESTs) and the transformation of estrone sulfate into estrone by steroid sulfatase (STS).[52][53] The metabolic clearance rates and hence blood half-lives of estrogen conjugates like estrone sulfate are far longer than those of estradiol and estrone.[9][11][45] Estrogen conjugates, primarily estrone sulfate, serve as a large circulating reservoir for estradiol, and because of this, they function to greatly extend the biological half-life of oral estradiol.[9][11] As such, the biological half-life of oral estradiol is a composite parameter that is dependent on interconversion between estradiol and estrogen conjugates, as well as on enterohepatic recirculation.[11] Whereas the biological half-life of estradiol given by intravenous injection is only about 1 to 2 hours, the biological half-life of oral estradiol has a range of 13 to 20 hours due to the large and long-lasting pool of estrogen conjugates that is formed during first-pass metabolism and that serves to continuously replenish circulating estradiol levels.[11][9]
First-pass effect and differences from other routes
The first-pass effect that occurs with oral estradiol results in unusually high levels of estrone and estrogen conjugates in the circulation as well as of estradiol in the liver.[9] These unique properties of oral estradiol result in a number of pharmacological differences relative to the other routes of administration of estradiol.[9]
The high levels of estrone and estrogen conjugates that occur with oral estradiol raise the question of the pharmacodynamic significance of these metabolites.[9] In contrast to estradiol however, estrone has very low activity as an estrogen.[9][54][55] The affinities of estrone for the human ERs and its estrogenic activity have been reported to be approximately 3 to 4% of those of estradiol.[9] In addition, unlike estradiol and estriol, estrone is not accumulated in target tissues.[9] Because estrone can be transformed into estradiol, most of its activity in vivo is actually due to conversion into estradiol.[9] In accordance, dosages of oral and transdermal estradiol that achieve similar levels of estradiol have been found, in spite of markedly elevated levels of estrone with oral estradiol but not with transdermal estradiol, to possess equivalent and non-significantly different potency in terms of clinical measures including suppression of LH and FSH levels, inhibition of bone resorption, and relief of menopausal symptoms such as hot flashes.[9][42][56][57][50] In addition, estradiol levels were found to correlate with these effects, while estrone levels did not.[42][56] These findings suggest that estrone contributes very little or not at all to the estrogenic potency of estradiol, while also not antagonizing the estrogenic activity of estradiol.[9][42][56][57] This contradicts some in vitro research suggesting that estrone might be able to partially antagonize the actions of estradiol.[58][59][60]
On the other hand, it has been suggested by some authors that the high levels of estrone and/or estrone conjugates with oral estradiol may result in excessive estradiol levels in certain tissues such as the breasts and endometrium, due to high expression in these tissues of the requisite enzymes (17β-HSDs and STS) necessary to transform these metabolites back into estradiol.[48][45][63][64] In accordance, circulating levels of estrone sulfate have been found to be positively associated with breast density in postmenopausal women treated with oral estradiol, with 1.3% higher breast density observed for every 1 ng/mL greater level of estrone sulfate.[65][66] Similarly, levels of estradiol, estrone, and estrone sulfate are all strongly associated with the risk of breast cancer in women.[65] Preclinical studies have shown that estrone sulfate, via local transformation into estradiol, stimulates the growth of mammary cancer cells.[67][68]
Due to the first pass through the liver, disproportionate and supraphysiological levels of estrogens occur locally in the liver with oral estradiol.[69][11] These levels are approximately 4- to 5-fold higher than in the circulation, based on differences in hepatic estrogenic potency for oral estradiol relative to transdermal estradiol.[69][9] As a result, there is abnormally high estrogenic signaling in the liver with oral estradiol, and a variety of unphysiological effects on liver protein synthesis result.[9][11] Through modulation of liver protein synthesis, oral estradiol increases the risk of blood clots,[70] increases circulating levels of a variety of binding proteins including thyroid binding globulin (TBG), cortisol binding globulin (CBG), sex hormone binding globulin (SHBG), growth hormone binding protein (GHBP),[71] insulin-like growth factor-binding proteins (IGFBPs),[72] and copper binding protein (CBP),[43][73] suppresses growth hormone (GH)-mediated insulin-like growth factor 1 (IGF-1) production,[74][75] and produces positive blood lipid changes, among a variety of other effects.[44][76][11] In contrast to oral estradiol, transdermal estradiol has relatively minimal impact on liver protein synthesis.[9] As an example, a study found that 1 mg/day oral estradiol significantly increased SHBG levels by 45%, while 50 µg/day transdermal estradiol increased SHBG levels non-significantly by only 12%.[77][78][79]
In the circulation, approximately 38% of estradiol is reversibly bound to SHBG and 60% is reversibly bound to albumin in women under normal physiological circumstances, with 2 to 3% of total estradiol circulating free or unbound at any given time.[3][2][1] Only estradiol that is free or unbound is able to be enter target cells and hence is biologically active.[1][11]:249[17] The increase in SHBG levels with oral estradiol (e.g., +50%) can result in a clinically meaningful increase in the fractions of sex hormones like estradiol and testosterone that are bound to SHBG, whereas this is not the case with typical clinical dosages of transdermal estradiol.[80][17] The increase in the fraction of estradiol bound to SHBG results in a significant decrease in the percentage of free or unbound and hence bioactive estradiol.[1][17] As a result, the bioavailability and potency of oral estradiol may be diminished relative to parenteral estradiol routes by some amount.[17][1] However, a study found that the free fraction of estradiol was similar with doses of oral and topical estradiol that resulted in equivalent total estradiol levels.[81]
Experimental oral formulations
Estradiol decanoate, estradiol cyclooctyl acetate, and EC508 (estradiol 17β-(1-(4-(aminosulfonyl)benzoyl)-L-proline)) are novel oral forms of estradiol that have been developed with improved properties, such as greater bioavailability and reduced first-pass effect.[82][83][84][85][86][87][88][89][90][91] Estradiol decanoate and estradiol cyclooctyl acetate were studied for potential use in menopausal hormone therapy and birth control pills but were never marketed,[82][83][84][85][86][87][88][89] while EC508 is currently under active development for use in menopausal hormone therapy.[90][91]
Graphs
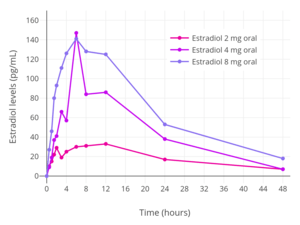 Estradiol levels after a single oral dose of 2, 4, or 8 mg estradiol in premenopausal women.[38]
Estradiol levels after a single oral dose of 2, 4, or 8 mg estradiol in premenopausal women.[38]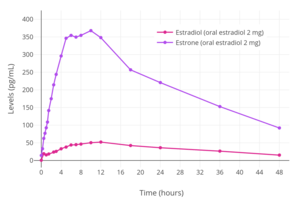 Estradiol and estrone levels following a single 2 mg dose of oral estradiol in postmenopausal women.[92]
Estradiol and estrone levels following a single 2 mg dose of oral estradiol in postmenopausal women.[92]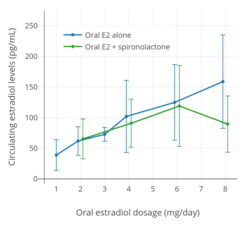 Mean estradiol levels during 1 to 8 mg/day oral estradiol therapy alone or in combination with 100 to 200 mg/day spironolactone in transgender women.[39]
Mean estradiol levels during 1 to 8 mg/day oral estradiol therapy alone or in combination with 100 to 200 mg/day spironolactone in transgender women.[39]
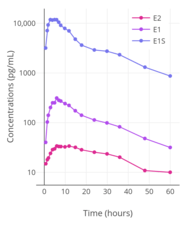 Levels of estradiol (E2), estrone (E1), and estrone sulfate (E1S) following a single 2 mg oral dose of micronized estradiol in postmenopausal women.[94] Peak levels of estradiol, estrone, and estrone sulfate were around 35, 300, and 12,000 pg/mL, respectively.[94]
Levels of estradiol (E2), estrone (E1), and estrone sulfate (E1S) following a single 2 mg oral dose of micronized estradiol in postmenopausal women.[94] Peak levels of estradiol, estrone, and estrone sulfate were around 35, 300, and 12,000 pg/mL, respectively.[94]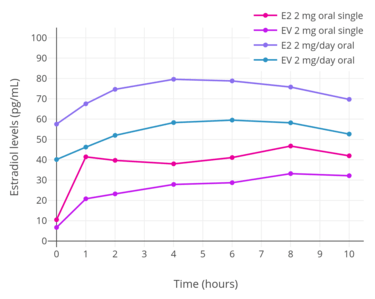 Estradiol levels after a single dose of 2 mg oral estradiol or 2 mg oral estradiol valerate and with continuous administration of 2 mg/day oral estradiol or 2 mg/day oral estradiol valerate (at steady state) in postmenopausal women.[95]
Estradiol levels after a single dose of 2 mg oral estradiol or 2 mg oral estradiol valerate and with continuous administration of 2 mg/day oral estradiol or 2 mg/day oral estradiol valerate (at steady state) in postmenopausal women.[95]
Buccal administration
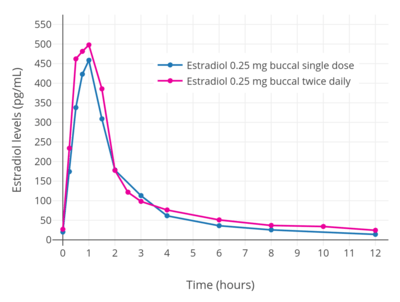
Estradiol has been studied for use by buccal administration.[9][97][96][98][99][100][101][102] Preclinical studies of buccal estradiol have also been conducted.[103][104][105][106] Buccal and sublingual administration of estradiol have similar characteristics.[9]
Administration of a troche (lozenge) containing 0.25 mg estradiol via the buccal route resulted in peak estradiol levels of about 450 pg/mL at 1 hour post-dose in postmenopausal women.[9][96] Following this, estradiol levels decreased to about 60 pg/mL at 4 hours post-dose and to about 15 pg/mL at 12 hours post-dose.[9][96] With continuous twice daily administration of 0.25 mg estradiol (0.5 mg/day total) via the buccal route once every 12 hours, peak estradiol levels at steady state after the last dose were about 500 pg/mL.[9][96]
Sublingual administration
Estradiol tablets can be taken sublingually instead of orally.[9][107][108] Non-micronized estradiol tablets in doses of 0.125, 0.25, and 1 mg were previously marketed for use by sublingual administration under brand names such as Diogynets and Estradiol Membrettes in the 1950s.[109][110][111][112] Non-micronized estradiol has poor water solubility, but micronized estradiol is rapidly absorbed by the sublingual route.[107] All oral estradiol tablets are micronized, as this improves the efficiency of estradiol absorption in the gastrointestinal tract.[21] Likewise, all oral estradiol valerate tablets seem to be micronized.[26] The sublingual route is, in actuality, probably a combination of sublingual and oral delivery of estradiol due to incidental swallowing of some of the estradiol.[49]
The absorption of sublingual estradiol can be attributed to the rich vascularization under the tongue.[107] With administration of an oral estradiol tablet sublingually, complete dissolution of the tablet occurs within a few minutes and circulating levels of estradiol begin to rise within 5 minutes.[107] Maximal levels of estradiol occur after 30 to 60 minutes of administration.[107] After this, estradiol levels drop steeply within 4 hours, and this is followed by a more gradual decline in levels of estradiol and a return to baseline concentrations by 24 hours.[107] The rapid rise and steep fall of estradiol levels with sublingual administration of estradiol is analogous to the case of intravenous injection and intranasal administration of the hormone.[9][11][4]
Sublingual administration of medications that are subject to a high first-pass effect with oral administration can result in improved bioavailability because the first pass through the intestines and liver is bypassed.[107] As a result, sublingual estradiol has been found to result in estradiol levels and a ratio of estradiol to estrone that are substantially higher than oral estradiol.[9][107][113] Maximal circulating levels of estradiol are as much as 10-fold higher with sublingual administration than with oral administration, and the absolute bioavailability of estradiol is approximately 5-fold higher.[9][107] On the other hand, levels of estradiol fall rapidly with sublingual administration, whereas they remain elevated for a prolonged period of time with oral administration.[9][11] This is due to the large circulating pool of hormonally inert estrogen conjugates with long half-lives that is reversibly generated with oral estradiol during first-pass metabolism, which serves as a metabolism-resistant and long-lasting reservoir for continuous reconversion back into estradiol.[9][11] It is also responsible for the differences in ratios between sublingual estradiol and oral estradiol in terms of maximal estradiol levels (10:1) achieved and absolute bioavailability (5:1).[9][11] A study in marmoset monkeys found that the bioavailability of sublingual estradiol was 10% of that of estradiol administered by intramuscular injection.[3]
After a dose of sublingual estradiol, levels of estrone start to slowly but progressively rise within 10 minutes.[107] Estrone levels surpass estradiol levels at around 2 hours post-dose and reach a maximum at about 4 hours.[107] It has been speculated that the high delayed levels of estrone with sublingual estradiol may be due to the rich lymphatic drainage in the neck region, which may result in estradiol being taken up by the reticuloendothelial system and then metabolized into estrone.[107]
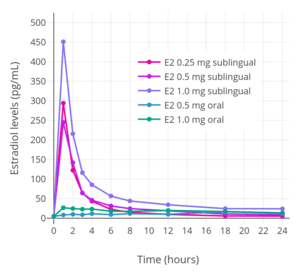
Sublingual administration of a single 0.25 mg tablet of micronized estradiol has been found to produce peak levels of 300 pg/mL estradiol and 60 pg/mL estrone within 1 hour.[9] A higher dose of 1 mg estradiol was found to result in maximum levels of 450 pg/mL estradiol and 165 pg/mL estrone, which was followed by a rapid decline in estradiol levels to 85 pg/mL within 3 hours.[9] Conversely, the decline in estrone levels was much slower and reached a level of 80 pg/mL after 18 hours.[9] A single administration of 4 mg micronized estradiol (two 2-mg Estrace tablets) under the tongue, considered a very high dose of sublingual estradiol, has been found to result in maximal levels of estradiol of 1759 ± 704 pg/mL, with a range of 634 to 2840 pg/mL, after 1 hour in a mixed group of normotensive and hypertensive postmenopausal women.[114] A replication of this study using the same dosage and protocols measured estradiol levels of 2227 ± 1180 pg/mL for the whole group of women but found that estradiol levels between the normotensive and hypertensive groups were significantly different at 1790 ± 869 pg/mL and 2664 ± 1490 pg/mL, respectively.[115][116]
Although sublingual administration of estradiol has a relatively short duration, the medication can be administered multiple times per day in divided doses to compensate for this.[9][117][118] Studies that used high doses of sublingual estradiol in the treatment of severe postpartum depression have administered a dose of 1 mg 3 to 8 times per day.[119][120][117][118] In one study, which administered a mean total dosage of sublingual estradiol of 4.8 mg/day, estradiol levels remained elevated at about 130 pg/mL on average in the morning before the first dose of the day.[119]
Oral micronized estradiol valerate tablets can be taken sublingually as well.[121][122] The administration of 2 mg oral micronized estradiol valerate tablets (Progynova, Schering) sublingually 3 or 4 times per day resulted in circulating estradiol levels of about 290 pg/mL to 460 pg/mL in premenopausal women (time of measurements not given).[121][122] Steady-state levels of estradiol were achieved within about 5 or 6 days.[121][122] Levels of progesterone, luteinizing hormone, and follicle-stimulating hormone were all considerably suppressed, and ovulation, as well as the associated mid-cycle hormonal surges, were prevented.[121][122] Sublingual estradiol valerate is used for cycle control in egg donation and surrogacy in cisgender women and is used in hormone therapy for transgender women.[121][122][123]
Cyclodextrin-containing formulations of sublingual estradiol with improved water solubility and absorption have been developed and studied.[124][125][126][127][128]
Clinical effects
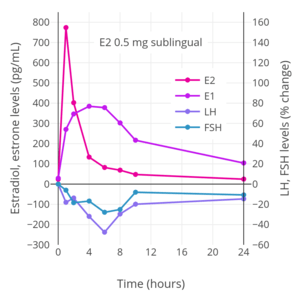
The total endometrial proliferation dose of sublingual estradiol in women is 60 to 140 mg per cycle or 14 days and of sublingual estradiol benzoate in women is 60 to 180 mg per cycle or 14 days.[129]:310 Both sublingual estradiol and sublingual estradiol benzoate have a persistence of estrogenic effect after a dose of only one day.[129]:310 The effects of sublingual estradiol on gonadotropin levels have also been studied in postmenopausal women.[107][130][108][131] After a dose of sublingual estradiol, levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decrease precipitously within 4 hours.[107] Following this, LH and FSH levels gradually increase, and return to near-baseline levels by 24 hours.[107] One study found no difference between oral and sublingual estradiol in suppression of LH levels.[107] However, FSH levels were suppressed to a greater extent with sublingual estradiol than with oral estradiol in the study.[107]
It is notable that the magnitude of the genomic effects of estradiol (i.e., signaling through the nuclear ERs) may, at least in some cases, be dependent on the total estrogenic exposure as opposed to the duration of exposure.[9] For instance, in normal human epithelial breast cells and ER-positive breast cancer cells, the rate of breast cell proliferation has been found not to differ with estradiol incubation of 1 nM for 24 hours and incubation of 24 nM for 1 hour.[9] In other words, short-term high concentrations and long-term low concentrations of estradiol appear to have the same degree of effect in terms of genomic estrogenic signaling, at least in terms of breast cell proliferation over a 24-hour period.[9] On the other hand, non-genomic actions of estradiol, such as signaling through membrane estrogen receptors like the GPER, may be reduced with short-term high concentrations of estradiol relative to more sustained levels.[9] For instance, although daily intranasal administration of estradiol is associated with comparable clinical effectiveness (e.g., for hot flashes) relative to longer acting routes of estradiol administration in postmenopausal women, it is also associated with significantly lower rates of breast tension (tenderness and enlargement) relative to longer acting estradiol routes, and this is thought to reflect comparatively diminished non-genomic signaling.[9]
Graphs
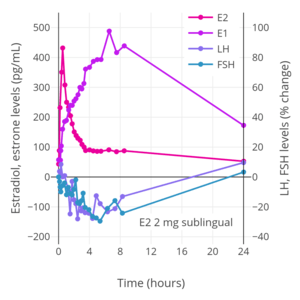
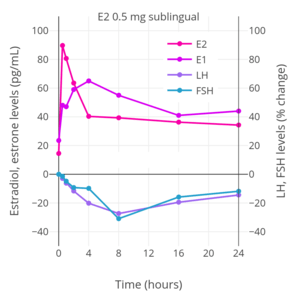
_taken_sublingually_in_premenopausal_women.png) Hormone levels with 2 mg oral micronized estradiol valerate tablets (Progynova, Schering) taken 3 or 4 times per day (6–8 mg/day total) sublingually (SL) in premenopausal women.[121][122] Time of blood collection after medication administration was not specified.[121][122] Source was Serhal et al. (1989, 1990).[121][122]
Hormone levels with 2 mg oral micronized estradiol valerate tablets (Progynova, Schering) taken 3 or 4 times per day (6–8 mg/day total) sublingually (SL) in premenopausal women.[121][122] Time of blood collection after medication administration was not specified.[121][122] Source was Serhal et al. (1989, 1990).[121][122]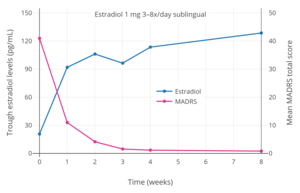 Trough estradiol levels and MADRS scores with 1 mg sublingual micronized estradiol 3 to 8 times per day (3 to 8 mg/day total; mean 4.8 mg/day total) in women with postpartum depression.[117] Blood was drawn specifically in the mornings before the first dose of sublingual estradiol for the day.[117] Source was Akohas et al. (2001).[117]
Trough estradiol levels and MADRS scores with 1 mg sublingual micronized estradiol 3 to 8 times per day (3 to 8 mg/day total; mean 4.8 mg/day total) in women with postpartum depression.[117] Blood was drawn specifically in the mornings before the first dose of sublingual estradiol for the day.[117] Source was Akohas et al. (2001).[117]
Intranasal administration
Estradiol has been studied and used by intranasal administration.[97][9] It was available as a cyclodextrin-containing nasal spray under the brand name Aerodiol in some countries,[134][135][136][137] although this specific product was discontinued in 2007.[138][139] The product was administered once per day as one 150-μg spray in each nostril (300 μg/day total).[140] Intranasal estradiol has pharmacokinetics similar to those of sublingual and intravenous administration of estradiol, including a sharp peak and then rapid decline in estradiol levels.[9] Despite the relatively short duration of intranasal estradiol, it has similar effectiveness to other, longer-lasting routes of administration in terms of relief of menopausal symptoms like hot flashes.[9]
Transdermal administration
Transdermal estradiol is available in the forms of patches, gels, emulsions, and sprays.[141][142][9][17][143] In the case of gels, emulsions, and sprays, the route is sometimes referred to as topical rather than as transdermal.[142][144][5] Topical administration can also refer to vaginal administration of gels and creams however.
Estradiol has moderate skin permeability, which is based on the lipophilicity and hydrophilicity of a compound.[9][145] In general, the more polar groups, such as hydroxyl groups, that are present in a steroid, and hence the more hydrophilic and less lipophilic it is, the lower its skin permeability.[9][145] For this reason, estrone and progesterone have higher skin permeability, while estriol and cortisol have lower skin permeability.[9] The transdermal bioavailability of estradiol in an alcohol solution is approximately 10%.[146][145] Transdermal estradiol reservoir patches have been reported to have a bioavailability of 3 to 5%.[147] Estradiol is a highly potent compound and circulates at picomolar concentrations (pg/mL), which makes it ideal for transdermal application as only small amounts of substance need to be delivered across the skin.[50] Conversely, progesterone, which circulates at levels in the nanomolar range and requires a far higher quantity of substance for biological effect, is not well-suited for transdermal delivery.[50]
Regardless of administration form, such as patch or gel, transdermal estradiol is transported into the skin, including through the stratum corneum, epidermis, and dermis, by a passive diffusion process.[9][148] Following this, estradiol is then taken up by local capillary blood vessels and delivered into the circulation.[9] There is a depot effect in the skin with transdermal estradiol, which results in continuous delivery of transdermal estradiol into the circulation.[17][148] This is because the skin functions as a semipermeable membrane and there is a concentration gradient between the application site of transdermal estradiol and capillary blood, with the rate of diffusion of estradiol across the stratum corneum being the specific rate-limiting factor in absorption.[9][148] As a result, peaks and troughs in circulating estradiol levels are limited, and the skin and subcutaneous fat act as a reservoir of estradiol that maintains circulating estradiol levels between doses.[17] For these reasons, transdermal estradiol can provide near-constant circulating levels of estradiol, similarly to oral estradiol.[17][9] Enzymes that metabolize estradiol are minimally expressed in the skin, and for this reason, the metabolism of estradiol in the skin is low.[9]
The site of application of transdermal estradiol can influence its bioavailability.[50] A study found comparable absorption of transdermal estradiol patches (within ±25% of reference) for a number of skin sites including the abdomen, upper arm, upper thigh, lower back, and side.[149][150] However, absorption was 15% lower for the upper thigh compared to the abdomen and the difference was significant.[151][150] Another study found that transdermal estradiol patches had 20 to 25% higher bioavailability when applied to the buttocks than when applied to the abdomen.[50] Studies of topical steroids have found that the scrotum is especially permeable among skin sites.[152] Studies of transdermal testosterone cream, gel, and patches applied to the scrotum in men have observed 5- to 8-fold higher levels of testosterone than with application to conventional skin sites.[153][154] In a study of topical application of hydrocortisone solution in men, skin permeability relative to the forearm (1.0) was 42.0 for the scrotum, 13.0 for the jaw angle, 6.0 for the forehead, 3.6 for the underarm, 3.5 for the scalp, 1.7 for the back, 0.8 for the palm of the hand, 0.4 for the ankle, and 0.1 for the sole of the foot.[152][155][156][157] In accordance with findings with other topical steroids, a study in men with prostate cancer treated with transdermal estradiol patches applied to the scrotum observed about 5-fold higher estradiol levels relative to application to conventional skin sites such as the forearm.[158][159] Penile skin may have similarly enhanced absorption characteristics relative to scrotal skin.[160]
Transdermal estradiol bypasses the intestines and liver and hence the first-pass metabolism that is associated with oral administration.[9][50] In addition, unlike oral estradiol, transdermal estradiol is not associated with supraphysiological concentrations of estrone or estrogen conjugates like estradiol sulfate, and transdermal estradiol does not have disproportionate effects on liver protein synthesis.[9][50] In accordance, estradiol, at typical menopausal replacement dosages, has been found not to increase the risk of blood clots or insulin resistance,[70][11] nor to affect hepatic SHBG, IGF-1, GHBP,[71] IGFBP,[72] and other protein production and by extension circulating hepatic protein levels.[74][75][73][50] However, at higher doses, transdermal estradiol has been associated with a significantly higher incidence of stroke in postmenopausal women, probably due to blood clots.[161][162] Another larger study did not find a significantly higher risk of blood clots with similar doses of transdermal estradiol however.[163]
Transdermal patches

Estradiol patches have an extended duration and are available for twice-weekly (3–4-day) and once-weekly (7-day) application, while gels, emulsions, and sprays are administered daily.[142][15][9][164] There are two types of estradiol patches: reservoir patches, which have been described as first-generation patches, and matrix patches, which are considered to be improved second-generation patches.[9][11][142] Reservoir patches were designed for twice-weekly application, while matrix patches have been produced for both twice-weekly and once-weekly application.[11] Reservoir patches of estradiol (e.g., Estraderm) are mostly no longer used, with most estradiol patches available today being matrix patches (e.g., Alora, Climara, Esclim, Estradot, FemPatch, Menostar, Oesclim, Vivelle, and Vivelle-Dot).[142]
A dosage of 1 mg/day oral estradiol is considered to be roughly equivalent to 50 µg/day transdermal estradiol and a dosage of 2 mg/day oral estradiol is considered to be equivalent to 100 µg/day transdermal estradiol.[51][11][9] Estradiol patches delivering a daily dosage of 0.05 mg (50 µg) achieve mean estradiol and estrone levels of 30 to 65 pg/mL and 40 to 45 pg/mL, respectively, while a daily dosage of 0.1 mg (100 µg) attains respective mean levels of 50 to 90 pg/mL and 30 to 65 pg/mL of estradiol and estrone.[15] In general, Climara-type estradiol transdermal patches have an approximate 1:1 ratio of estradiol delivered in μg/day relative to circulating estradiol concentration in pg/mL.[159] In other words, a 100 μg/day Climara estradiol patch may be expected to produce circulating estradiol levels of around 100 pg/mL.[159] Transdermal estradiol patches produce an estradiol to estrone ratio of about 1:1.[9][11] Following removal of an estradiol patch, circulating estradiol levels decrease to baseline within 24 hours.[9]
Typical dosages of estradiol patches are intended to provide the minimum amount of estrogen replacement necessary for the effective alleviation of menopausal symptoms, and for this reason, they achieve relatively low levels of estradiol.[9] A dosage of two to six 100 µg/day transdermal estradiol patches can achieve mean levels of estradiol in the area of 200 to 400 pg/mL and can be used as a form of high-dose estrogen therapy, for instance to suppress testosterone levels in the treatment of prostate cancer in men and in feminizing hormone therapy for transgender women.[13][165][166] High-dose transdermal estradiol patches have also been studied in the treatment of postpartum depression and postpartum psychosis; in one such study, 200, 400, and 800 μg/day estradiol in the form of transdermal patches resulted in estradiol levels of 286 pg/mL, 675 pg/mL, and 1032 pg/mL, respectively.[167] In another study, estradiol levels with 800 μg/day estradiol in the form of transdermal patches (Estraderm TTS) resulted in estradiol levels of 690 to 815 pg/mL.[168] However, there is erratic absorption and considerable variation in estradiol levels using high-dose estradiol patches both between and within individuals, with one study finding that estradiol levels ranged from 70 pg/mL to 1,045 pg/mL.[169]
The Prostate Adenocarcinoma: TransCutaneous Hormones (PATCH) study is a randomized controlled trial of high-dose transdermal estradiol patches versus gonadotropin-releasing hormone agonist monotherapy in the treatment of prostate cancer in approximately 2,200 men.[170][171][172] It is specifically comparing three to four 100 μg/day estradiol patches (FemSeven) against goserelin implants.[170] The study was started in March 2006 and is estimated for completion in August 2021.[170] Its objectives include comparison of survival, cardiovascular mortality and morbidity, pharmacological activity (e.g., suppression of testosterone levels), other side effects and toxicities, and quality of life.[170] In addition to the PATCH trial, the Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE) study added a high-dose estradiol patches arm (~2,000 men) in July 2017.[173][171][172]
Estradiol patches are associated with local skin reactions and such as irritation in 14.2% of individuals (with reservoir patches), mild-to-moderate erythema (redness) in 50 to 60% of individuals, and allergic reactions due to cutaneous sensitization.[9][11] Up to 5% of people using reservoir patches may discontinue therapy due to skin reactions.[11] Visible adhesive residues are also often left by estradiol patches following their removal.[9] Transdermal estradiol gel can serve as an alternative to transdermal estradiol patches for individuals who experience intolerable skin reactions with them.[174] Estradiol patches should not be applied to the breast as this may result in high local levels of estradiol in the breasts and hence an increased likelihood of breast tenderness.[175]
| Brand name |
Forms (µg/day) | Duration | Type | Size (cm2)a | Estradiol (mg) | Levels (pg/mL) |
Launch (year) |
Hits | |
|---|---|---|---|---|---|---|---|---|---|
| Alora | 25, 50, 75, 100 | 3–4 days | Matrix | 9, 18, 27, 36 | 0.77, 1.5, 2.3, 3.1 | 43–144 | 1996 | 42,300 | |
| Climara | 25, 37.5, 50, 60, 75, 100 | 7 days | Matrix | 6.5, 9.375, 12.5, 15, 18.75, 25 | 2, 2.85, 3.8, 4.55, 5.7, 7.6 | 17–174 | 1994 | 110,000 | |
| Climara Prob | E2 (45) + LNG (15) | 7 days | Matrix | 22 | 4.4 | 27–54 | 2003 | 23,400 | |
| CombiPatchb | E2 (50) + NETA (14, 25) | 3–4 days | Matrix | 9, 16 | 0.62, 0.51 | 27–71 | 1998 | 33,500 | |
| Estradiolc | 25, 37.5, 50, 75, 100 | 3–4 days | Matrix | 2.5, 3.75, 5, 7.5, 10 | 0.41, 0.62, 0.82, 1.23, 1.64 | 30–145 | 1996 | – | |
| Estradiolc | 25, 37.5, 50, 75, 100 | 7 days | Matrix | 7.75, 11.625, 15.5, 18.6, 23.25, 31 | 0.97, 1.46, 1.94, 2.33, 2.91, 3.88 | 17–174 | 2000 | – | |
| Menostar | 14 | 7 days | Matrix | 3.25 | 1 | 13–21 | 2004 | 21,300 | |
| Minivelle | 25, 37.5, 50, 75, 100 | 3–4 days | Matrix | 1.65, 2.48, 3.3, 4.95, 6.6 | 0.41, 0.62, 0.83, 1.24, 1.65 | 30–117 | 2012 | 15,100 | |
| Vivelle | 3–4 days | Matrix | 30–145 | 2000 | 91,900 | ||||
| Vivelle-Dot | 25, 37.5, 50, 75, 100 | 3–4 days | Matrix | 2.5, 3.75, 5, 7.5, 10 | 0.39, 0.585, 0.78, 1.17, 1.56 | 30–145 | 1996 | 68,900 | |
| Abbreviations: E2 = Estradiol. LNG = Levonorgestrel. NETA = Norethisterone acetate. EtOH = Ethanol. Notes: | |||||||||
_in_postmenopausal_women.png)
_in_postmenopausal_women.png)
_in_women.png) Levels of estradiol over a period of 8 days after a single application of a 50 or 100 μg/day Climara-type (Climara, Menostar, Mylan generic) once-weekly transdermal estradiol matrix patch to the abdomen and removed on day 7 in postmenopausal women.[182]
Levels of estradiol over a period of 8 days after a single application of a 50 or 100 μg/day Climara-type (Climara, Menostar, Mylan generic) once-weekly transdermal estradiol matrix patch to the abdomen and removed on day 7 in postmenopausal women.[182]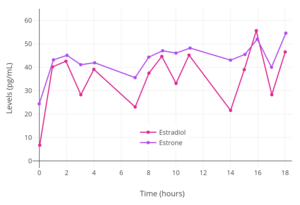 Levels of estradiol and estrone with application of a single 50 µg/day estradiol transdermal reservoir patch (Estraderm) in postmenopausal women.[144]
Levels of estradiol and estrone with application of a single 50 µg/day estradiol transdermal reservoir patch (Estraderm) in postmenopausal women.[144]_with_and_without_an_ethanol_injection_in_postmenopausal_women.png) Levels of estradiol over the course of 15 days with a single application of a 100 µg/day estradiol transdermal reservoir patch (Estraderm) in postmenopausal women.[17] This patch has a 3- to 4-day duration and is designed for twice-weekly use. Patch was applied on day 1 and removed on day 7.[17] In one group, ethanol was injected into the area between the patch and the skin on day 3 to restore the solvent, and resulted in increased bioavailability and a prolonged duration of the patch.[17]
Levels of estradiol over the course of 15 days with a single application of a 100 µg/day estradiol transdermal reservoir patch (Estraderm) in postmenopausal women.[17] This patch has a 3- to 4-day duration and is designed for twice-weekly use. Patch was applied on day 1 and removed on day 7.[17] In one group, ethanol was injected into the area between the patch and the skin on day 3 to restore the solvent, and resulted in increased bioavailability and a prolonged duration of the patch.[17]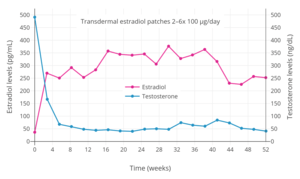
Transdermal gel
Estradiol is available as a transdermal gel in the form of gel dispensers and gel packets. Major estradiol gel dispenser products include EstroGel and Elestrin while major estradiol gel packet products include DiviGel and Sandrena. Estradiol gels are administered daily.[142][15][9][164] When estradiol is administered as a hydroalcoholic gel, it dries within 2 to 5 minutes following application to the skin.[148] A single application of a transdermal estradiol gel results in a sustained increase in estradiol levels for at least 24 hours.[17][148] The apparent elimination half-life of estradiol with transdermal estradiol gel is 36 hours.[148]
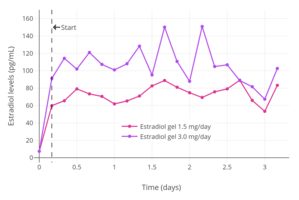
Once daily application of 1.25 g topical gel containing 0.75 mg estradiol (brand name EstroGel) for 2 weeks was found to produce mean peak estradiol and estrone levels of 46.4 pg/mL and 64.2 pg/mL, respectively.[148] The time-averaged levels of circulating estradiol and estrone with this formulation over the 24-hour dose interval were 28.3 pg/mL and 48.6 pg/mL, respectively.[148] Levels of estradiol and estrone are stable and change relatively little over the course of the 24 hours following an application, indicating a long duration of action of this route.[148] Steady-state levels of estradiol are achieved after 3 days of application.[148] A higher dosage of estradiol gel containing 1.5 mg estradiol per daily application has been found to produce mean estradiol levels of 40 to 100 pg/mL and estrone levels of 90 pg/mL, while 3 mg per day has been found to result in respective mean estradiol and estrone levels of 60 to 140 pg/mL and 45 to 155 pg/mL.[15] Topical estradiol gel at a dosage of 3 mg/day has been reported to be equipotent with 2 mg oral estradiol in terms of therapeutic effects and FSH suppression, as well as to produce similar estradiol levels.[81] Transdermal estradiol gel produces an estradiol to estrone ratio of about 1:1.[9][11]
Transdermal estradiol gel can be used as a form of high-dose estrogen in transgender women.[174] However, the doses needed require application to a large surface of skin that amounts to the combined area of both legs for proper absorption.[174] As a result, high-dose transdermal estradiol gel is not a primary choice of estrogen therapy for most transgender individuals.[174] Similarly to transdermal estradiol patches, high-dose transdermal estradiol gel has been studied in the treatment of prostate cancer as well.[184][185][186][187][188][189][190] In these studies, levels of estradiol with estradiol gel or ointment were 84 pg/mL with 3 mg/day, 185 pg/mL with 6 mg/day, 107 pg/mL with 10 mg/day, and 473 pg/mL with 20 mg/day.[185][186][187][188][189][190]
Studies have found that topical application of estradiol to the breasts increases local levels of estradiol in breast tissue.[191][192][193][194]
The total endometrial proliferation dose of transdermal estradiol gel in women has been reported to be 150 mg per cycle or 14 days.[195][129]:310 However, it has also been found that 6 mg/day estradiol gel is effective for endometrial proliferation in women.[196]
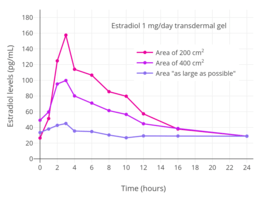 Estradiol levels after the last dose with 1 mg/day transdermal estradiol gel applied to different amounts of skin area (200 cm2, 400 cm2, or as large as possible) in postmenopausal women.[198]
Estradiol levels after the last dose with 1 mg/day transdermal estradiol gel applied to different amounts of skin area (200 cm2, 400 cm2, or as large as possible) in postmenopausal women.[198]
Other transdermal formulations
Estradiol is available in the form of transdermal emulsions and sprays as well.[143] Estradiol emulsions and sprays are administered daily.[142][15][9][164]
Variability in pharmacokinetics
Transdermal estradiol patches are described as delivering a fixed amount of estradiol such as 50 µg/day or 100 µg/day.[9] However, there is large interindividual variability and intraindividual variability`in the pharmacokinetic parameters of transdermal estradiol, and fluctuations in circulating estradiol levels with estradiol patches is almost as great as with oral estradiol.[9][50][11][17] As such, the actual delivery rate of estradiol and mean levels of estradiol achieved with transdermal estradiol patches may be different from what is described and from the mean levels observed in clinical studies, respectively.[9]
A wide range of estradiol levels are measured in women using the same estradiol patch or gel and dosage, with an up to about 10-fold difference in levels.[9][50][17] In a study of estradiol gel and patches, the maximal difference in peak levels between individuals was 11-fold for the gel and 7-fold for the patch, and the maximal difference in area-under-the-curve levels (total exposure) was 6-fold for the gel and 8-fold for the patch.[50] It has likewise been reported that the interindividual variability in bioavailability with Estraderm reservoir patches ranges from 25 to 225%.[17] In as many as 30% of women treated with a 50 µg/day estradiol patch, estradiol levels are low.[9] There are also significant short-term intraindividual differences in estradiol levels with estradiol patches; estradiol levels can fluctuate considerably from hour to hour.[9][144] In addition, estradiol levels with estradiol patches are higher in the evening than in the morning, which may be due to circadian variations in skin blood flow that may influence absorption.[9] In terms of area-under-the-curve levels of estradiol, the interindividual variability of transdermal estradiol has been found to be 20 to 44% using different transdermal formulations, and the intraindividual variability with transdermal estradiol has been found to be 20%.[11]
Factors which may contribute to inter- and intraindividual variability with transdermal estradiol include skin location and thickness; hair follicle density; solvent (alcohol) evaporation; skin dehydration, ambient temperature, and humidity; and reservoir size.[17]
Vaginal administration
Vaginal estradiol is available in the forms of tablets, creams, inserts or suppositories, and rings.[142][9][141] Vaginal estradiol tablets, creams, and inserts are usually administered once daily to twice weekly, whereas vaginal estradiol rings have a sustained action and are replaced once every 90 days.[142][9] Vaginal estradiol can be used both as a systemic form of estradiol therapy, and at very low doses to selectively achieve a local vaginal effect without systemic effects, for instance in the treatment of menopausal symptoms such as vaginal atrophy and dryness.[9][199]
Vaginal estradiol is rapidly and almost completely absorbed.[97] The absorption of vaginal estradiol is slightly greater in women with vaginal atrophy.[97] Vaginal estradiol has high bioavailability and greatly increased potency compared to oral estradiol, with about 10- to 20-fold the comparative potency of oral estradiol.[9] The greater potency of vaginal estradiol relative to oral estradiol is due to the lack of the first pass associated with oral estradiol and due to low local metabolism of estradiol in the vagina.[9] Because of the high estradiol levels achieved, LH and FSH are more strongly suppressed with vaginal estradiol than with other routes.[97]
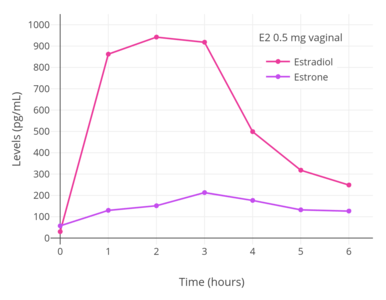
A daily dosage of 0.5 mg vaginal micronized estradiol has been found to result in estradiol and estrone levels of 250 pg/mL and 130 pg/mL, respectively.[15] Vaginal estradiol has a much higher estradiol-to-estrone ratio in comparison to oral estradiol.[9] The average ratio of estradiol to estrone with vaginal estradiol is 5:1, compared to 1:5 in the case of oral estradiol, a 10-fold difference.[9]
As vaginal estradiol is not subject to a first pass and bypasses the intestines and liver, it does not affect liver protein synthesis at menopausal replacement dosages, similarly to transdermal estradiol.[200]
Rectal administration
Estradiol has been assessed for use by rectal administration in a number of studies.[207][208][206][209][210] Uses of estradiol by this route have included treatment of menopausal symptoms in postmenopausal women.[207][208][206][209] Rectal administration of estradiol is described as qualitatively and quantitatively similar to vaginal administration of estradiol.[206][209][97] The use of estradiol by the rectal route considerably bypasses the liver and hence the first-pass metabolism that occurs with oral estradiol, similarly to other parenteral routes of estradiol such as vaginal and transdermal administration.[207][211] Irritation of the intestines does not usually occur with rectal estradiol.[206] The use of estradiol by the rectal route is not well-accepted by all individuals,[206] and due to its inconvenience, it has been said that rectal administration of estradiol has gained no practical clinical importance.[211]
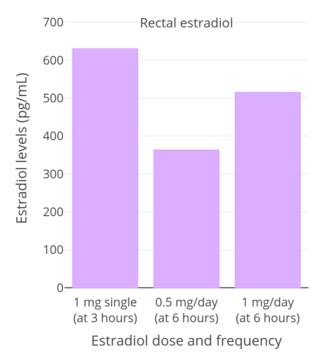
Lauritzen (1986) reported that 3 hours after a single rectal dose of 1 mg micronized estradiol, estradiol levels increased by 620 pg/mL and estrone levels increased by 120 pg/mL.[206][97] Subsequently, Lauritzen (1987, 1990) reported that 0.5 mg/day and 1 mg/day rectal estradiol resulted in respective estradiol levels of 363 pg/mL and 515 pg/mL 6 hours following the last dose.[207][208] These estradiol levels are fairly similar to those achieved by vaginal estradiol.[206][208][97] The estradiol-to-estrone ratio of rectal estradiol is about 5:1, which likewise is the same as that of vaginal estradiol.[207][206][97] Absorption of rectal estradiol occurs rapidly within 30 to 60 minutes, maximal estradiol levels occur at 3 hours post-dose, and circulating estradiol levels are reportedly maintained for 4 to 10 hours.[206][209][97] The duration of rectal estradiol is said to necessitate repeated administration 1 to 2 times per day.[206][209]
Rectal administration of estriol, which has similar properties to estradiol, has also been studied.[212] Administration of a rectal suppository containing 100 mg estriol resulted in estriol levels in pregnant women at term increasing by about 53%.[212] Estriol levels at term are normally between 5,000 and 20,000 pg/mL, which suggests that estriol levels may have increased following the suppository by about 5,000 to 10,000 pg/mL (precise levels were not provided).[213][214][215]
Intramuscular injection
Estradiol, in an ester prodrug form such as estradiol valerate or estradiol cypionate, can be administered by intramuscular injection, via which a long-lasting depot effect occurs.[216][8] In contrast to the oral route, the bioavailability of estradiol and its esters like estradiol valerate is complete (i.e., 100%) with intramuscular injection.[4] The levels of estradiol that are achieved with typical clinical dosages of injections are very high compared to other routes.[9][16][217][216][12] A single 1 to 10 mg dose of estradiol in oil solution by intramuscular injection has a duration of about 1 or 2 days.[211][218][219] Conversely, intramuscular injections of estradiol esters can have durations of days to months.[211]
| Estrogen | Form | Major brand name(s) | EPD (14 days) | CIC-D (month) | Duration |
|---|---|---|---|---|---|
| Estradiol | Oil solution | – | 40–60 mg | – | 1–10 mg ≈ 1–2 days |
| Aqueous suspensiona | Mego-E | ? | 3.5 mg | 3.5 mg ≈ >5 days | |
| Microspheres | Juvenum-E, Juvenum | ? | – | 1 mg ≈ 30 days | |
| Estradiol benzoate | Oil solution | Progynon-B | 25–35 mg | – | 5 mg ≈ 3–6 days |
| Aqueous suspension | Agofollin-Depot | 20 mg | – | 10 mg ≈ 16–21 days | |
| Estradiol dipropionate | Oil solution | Agofollin, Di-Ovocyclin, Progynon DP | 25–30 mg | – | 5 mg ≈ 5–8 days |
| Estradiol valerate | Oil solution | Delestrogen, Progynon Depot, Mesigyna | 20–30 mg | 5 mg | 5 mg ≈ 7–8 days; 10 mg ≈ 10–14 days; 40 mg ≈ 14–21 days; 100 mg ≈ 21–28 days |
| Estradiol cypionate | Oil solution | Depo-Estradiol, Depofemin | 20–30 mg | – | 5 mg ≈ 11–14 days |
| Aqueous suspensiona | Cyclofem, Lunelle | ? | 5 mg | 5 mg ≈ 14–24 days | |
| Estradiol benzoate butyratea | Oil solution | Redimen, Soluna, Unijab | ? | 10 mg | 10 mg ≈ 21 days |
| Estradiol enanthatea | Oil solution | Perlutal, Topasel, Yectames | ? | 5–10 mg | 10 mg ≈ 20–30 days |
| Estradiol undecylate | Oil solution | Delestrec, Progynon Depot 100 | ? | – | 10–20 mg ≈ 40–60 days; 25–50 mg ≈ 60–120 days |
| Polyestradiol phosphate | Aqueous solution | Estradurin | 40–60 mg | – | 40 mg ≈ 30 days; 80 mg ≈ 60 days; 160 mg ≈ 120 days |
| Estrone | Oil solution | Kestrin, Theelin | ? | – | 1–2 mg ≈ 2–3 days |
| Aqueous suspension | Estrone Aqueous Suspension | ? | – | ? | |
| Estriol | Oil solution | – | ? | – | 1–2 mg ≈ 1–4 days |
| Polyestriol phosphate | Aqueous solution | Gynäsan, Klimadurin, Triodurin | ? | – | 50 mg ≈ 30 days; 80 mg ≈ 60 days |
| Notes: All by intramuscular injection. All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/day (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate is 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Footnotes: a = Available only in combined injectable contraceptives (i.e., not available alone). Sources: See template. | |||||
Oil solution
A single 4 mg intramuscular injection of estradiol cypionate or estradiol valerate has been found to result in maximal plasma levels of estradiol of about 250 pg/mL and 390 pg/mL, respectively, with levels declining to 100 pg/mL (the baseline for estradiol cypionate) by 12 to 14 days.[16][217] A single 2.5 mg intramuscular injection of estradiol benzoate in patients being administered a GnRH analogue (and hence having minimal baseline levels of estrogen) was found to result in estradiol levels of more than 400 pg/mL at 24 hours post-administration.[216] The differences in the levels of estradiol achieved with these different estradiol esters may be explained by their different rates of absorption, as their durations and levels attained appear to be inversely proportional.[216] For instance, estradiol benzoate, which has the shortest duration (4 to 5 days with a single intramuscular injection of 5 mg), produces the highest levels of estradiol, while estradiol cypionate, which has the longest duration (~11 days with 5 mg), produces the lowest levels of estradiol.[216]
The duration of parenteral estradiol esters is dose-dependent.[220] With intramuscular injections of estradiol valerate, it is reported that a dose of 5 mg has a duration of 7 to 8 days, 10 mg a duration of 10 to 14 days, 40 mg a duration of 2 to 3 weeks (14 to 21 days), and 100 mg a duration of 3 to 4 weeks (21 to 28 days).[220][211][216]
A study of pseudopregnancy with intramuscular injections of 40 mg/week estradiol valerate and 250 mg/week hydroxyprogesterone caproate in women with estrogen deficiency observed estradiol levels of about 3,100 pg/mL at 3 months of therapy and 2,500 pg/mL at 6 months of therapy.[221]
| Estrogen | Dose | Peak levels | Time to peak | Duration |
|---|---|---|---|---|
| Estradiol benzoate | 5 mg | E2: 940 pg/mL E1: 343 pg/mL | E2: 1.8 days E1: 2.4 days | 4–5 days |
| Estradiol valerate | 5 mg | E2: 667 pg/mL E1: 324 pg/mL | E2: 2.2 days E1: 2.7 days | 7–8 days |
| Estradiol cypionate | 5 mg | E2: 338 pg/mL E1: 145 pg/mL | E2: 3.9 days E1: 5.1 days | 11 days |
| Notes: All via i.m. injection of oil solution. Determinations via radioimmunoassay with chromatographic separation. Sources: See template. | ||||
Aqueous suspension
Microcrystalline aqueous suspensions of estradiol esters, for instance of estradiol benzoate (brand names Agofollin Depot alone and Follivirin in combination with testosterone isobutyrate),[222][223] have been found to have longer duration of actions than oil solutions of the same esters when administered via intramuscular injection.[224][225][226][227][228][229][129]:310 Whereas the duration of a single intramuscular injection of amorphous estradiol benzoate in oil solution is 6 days, the duration of a single intramuscular injection of microcrystalline estradiol benzoate in aqueous suspension is 16 to 21 days.[129][225][230][231]
The duration of crystalline aqueous suspensions is highly dependent on crystal size.[232][233][228][234][235] Steroids and steroid fatty acid esters are lipophilic and have very low water solubility.[236] When they are suspended in the form of crystals in water, these crystals dissolve slowly, releasing steroid from their surfaces in the process.[236][237] The larger the particle sizes of the crystals, the slower the dissolution rate.[236] When a crystalline aqueous suspension of steroid is administered via intramuscular injection, a local crystalline depot suspended in local fluid is formed within the muscle.[236][237] These crystals slowly dissolve and the steroid is gradually absorbed into the body, resulting in the long duration of such preparations.[236][237]
Injection of crystalline aqueous suspensions require larger needle sizes than aqueous or oil solutions so that the crystals can pass through the needle lumen.[238] Crystals pose a risk of injection site reactions such as local irritation, pain, swelling, and redness.[238] The larger the crystal size, the more likely such reactions are to occur.
Microspheres
Estradiol is available in the form of an aqueous suspension of 1.0 mg estradiol encapsulated in microspheres for use by intramuscular injection once a month under the brand name Juvenum E in Mexico.[239][240] It achieves circulating estradiol levels of 163 pg/mL to 219 pg/mL in the first 3 to 12 hours following injection, which decrease to 42 to 66 pg/mL during the first 4 days post-injection and to 20 to 35 pg/mL after 8 days, with levels remaining in this range thereafter over 30 days.[239] These estradiol levels are similar to the normal levels that occur during the early follicular phase of the menstrual cycle in premenopausal women (24 to 75 pg/mL).[239] The elimination of the formulation follows three phases: a rapid phase in the first 2 days, a second phase during days 2 to 12 days with a biological half-life of 7 to 10 days, and a third phase in which estradiol levels remain elevated above baseline for up to 30 days.[239]
Polymers
Polyestradiol phosphate is an ester prodrug of estradiol in the form of a polymer which is used via intramuscular injection primarily to treat prostate cancer, but also to treat breast cancer and menopausal symptoms.[241][242] It has an extremely long duration of action, with an elimination half-life of about 70 days (10 weeks) following a single intramuscular administration of the medication.[243] In addition, unlike most other estradiol esters, the estradiol levels achieved with polyestradiol phosphate are highly constant and uniform.[243] Levels of estradiol in men with intramuscular injections of polyestradiol phosphate once every 4 weeks were about 350 pg/mL with 160 mg, 450 pg/mL with 240 mg, and almost 700 pg/mL with 320 mg, all measured after 6 months of treatment.[12] Polyestradiol phosphate has been discontinued in many countries, and remains available today in only a few countries, mostly the Nordic countries of Europe.[242][244]
Clinical effects
Estradiol valerate in oil solution by intramuscular injection has been studied in the treatment of prostate cancer.[245][246][247][248] Although parenteral estradiol has diminished effects on liver protein synthesis and by extension coagulation and cardiovascular risk compared to oral estradiol and non-bioidentical estrogens, a property attributable to its absence of disproportionate effects on the liver, sufficient doses of parenteral estradiol can nonetheless result in high estradiol concentrations in the liver and thereby increase coagulation and cardiovascular risk similarly.[129]:318 A single 10 to 20 mg intramuscular injection of estradiol valerate produces hemostasis and can be used to decrease blood flow during hemorrhage.[129] In accordance, 10 to 40 mg estradiol valerate by intramuscular injection once every 2 weeks in men with prostate cancer has been found to increase markers of coagulation and plasminogen system activation such as levels of thrombin–antithrombin complex and quantitative D-dimers.[245][246][248] Administration of daily prophylactic anticoagulation in the form of low molecular-weight heparin was able to successfully return these hemostasis markers to baseline in the men.[245][248]
Graphs
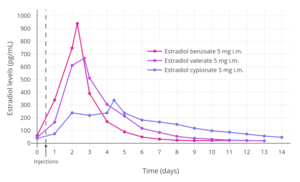
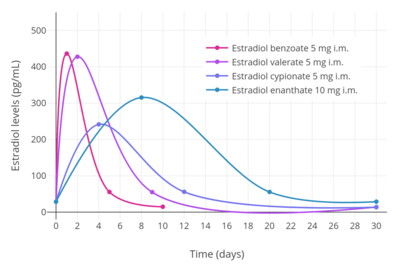 Idealized curves of estradiol levels after injection of different estradiol esters in women.[249]
Idealized curves of estradiol levels after injection of different estradiol esters in women.[249]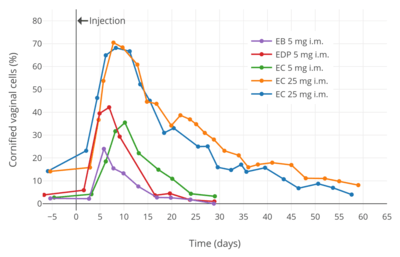
Subcutaneous injection
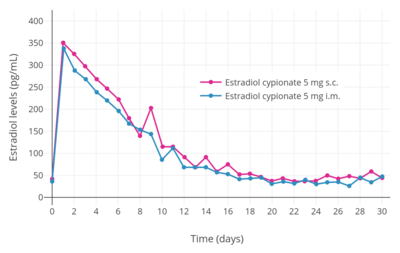
Estradiol esters like estradiol valerate and estradiol cypionate can be given by subcutaneous injection instead of intramuscular injection.[251] Subcutaneous and intramuscular injection of estradiol cypionate in an aqueous suspension has been found to result in levels of estradiol and other pharmacokinetic parameters (e.g., duration) that were virtually identical.[8] Studies have shown that subcutaneous injection of closely related steroid esters in oil like the androgen esters testosterone cypionate, testosterone enantate, and nandrolone decanoate is effective and has similar pharmacokinetics to intramuscular injection as well.[252][166][253][254][255][256][257] In addition, studies have found that many intramuscular injections are really subcutaneous injections, as individuals often do not actually penetrate deep enough to inject into muscle when attempting to perform an intramuscular injection and instead inject into the subcutaneous fat layer above the muscle.[258][259] This is particularly prevalent with injections into the buttocks and in overweight and obese individuals, due to the thicker layer of fat over muscle.[258][259] Subcutaneous injections of estradiol esters may be easier and less painful to perform than intramuscular injections, and hence may result in improved compliance and satisfaction with therapy.[8]
Subcutaneous implantation
Estradiol can be administered in a very long-lasting form via subcutaneous implantation of pure crystalline estradiol compressed into a small solid cylindrical pellet.[9][262] These pellets slowly and completely dissolve and are replaced once every 6 to 12 months, achieving high and very constant circulating levels of estradiol.[9][263][264] They are surgically inserted with the aid of a trocar by a trained physician in a medical office or clinic, and can be placed into locations including the lower abdomen, lower back, buttocks, or hips.[9][263][262] Subcutaneous pellets containing 20 mg estradiol (brand name Meno-Implant) or 25, 50, or 100 mg estradiol (brand name Estradiol Implants; discontinued) for replacement usually once every 6 months (range 4 to 8 months) are or have been available as approved pharmaceutical medications.[264] Up to 800 mg estradiol per implantation has been used.[265] Pharmaceutical estradiol pellet implants have been used almost exclusively in the United Kingdom, but have also been available in Australia and the Netherlands.[266][267] However, estradiol pellets have been discontinued in both the United Kingdom and Australia.[268][269] An estradiol implant has not been approved by the FDA as a pharmaceutical medication in the United States, but hormone pellet implants, including estradiol pellets, are available as custom compounded products in this country.[270][271][272]
Estradiol pellet implants are advantageous in that some women seem to need higher levels of estradiol for adequate relief of menopausal symptoms, and subcutaneous estradiol pellets are easily able to achieve such levels.[264][9] Conversely, this is not necessarily the case with oral or transdermal estradiol.[264][9] Another major advantage of estradiol pellet implants is convenience and guaranteed compliance.[264] They also do not have the issues pertaining to first-pass metabolism and liver protein synthesis of oral estradiol.[264][9] A major disadvantage of estradiol pellet implants is that they cannot be easily removed should this be necessary.[264] There are also concerns about accumulation of estradiol levels with long-term repeated pellet implantation.[264][9] Estradiol levels may remain above baseline for a year or in some cases 3 to 4 years following the last pellet insertion.[264] During this time, progestogen therapy should be continued to avoid the risk of endometrial changes.[264][263] Regular monitoring of estradiol levels and adjustment of dosing is recommended during therapy with estradiol pellet implants.[264]
Tachyphylaxis of relief of vasomotor symptoms, or hot flashes returning even with normal or supraphysiological estradiol levels, may occur in a small subset of cases with estradiol pellet implants.[264][9][266][273][263] The reason for this is unknown, but has been hypothesized to be a paradoxical effect of the high levels of estradiol achieved and/or a result of receptor desensitization caused by the long-term gradually decreasing levels of estradiol.[264][9] Such symptoms have been said to occur once estradiol levels begin to decrease, although there are also reports of such symptoms occurring 3 to 16 weeks (1 to 4 months) after pellet insertion, when estradiol levels should still be constant.[264][9] Hot flashes have notably been reported in pregnant women, who have very high and constantly increasing levels of estradiol.[274] When recurrence of hot flashes occurs with estradiol pellets, treated women often complain that their pellet has "run out".[264] Such symptoms can be temporarily offset with the use of supplemental oral or transdermal estradiol.[264]
Following insertion of an estradiol pellet, levels of estradiol rapidly increase, remain constant for about 4 months, and then gradually decrease.[264] A 25 mg subcutaneous estradiol pellet has been found to result in average estradiol levels of 90 pg/mL for 6 months, while two 25 mg pellets (50 mg total) resulted in estradiol levels of 180 pg/mL after 24 hours and levels of 100 to 120 pg/mL for 6 months.[9] Higher-dose pellets resulted in estradiol levels for 50 mg of 100 pg/mL, for 75 mg of 140 pg/mL, and for 100 mg of 150 pg/mL.[9] Estradiol levels are generally 50% higher than those of estrone, for an estradiol-to-estrone ratio of 1.5:1.[9] Very high levels of estradiol of between 400 and 1,000 pg/mL have been observed in a small subset of women treated with estradiol pellets and notably in those experiencing symptoms of tachyphylaxis.[264][9]
Estradiol pellet implants have been studied in the treatment of prostate cancer in men.[275][276][277][278][279]
Intrauterine administration
Intrauterine estradiol has been studied in the treatment of uterine hypoplasia in women.[280][281][227]
Intravenous injection
The administration of estradiol by intravenous injection has been studied.[38][282][283][284] It achieves extremely high peak levels of estradiol but has a very short duration.[38][282] Kuhnz et al. (1993) reported that a single intravenous injection of 0.3 mg estradiol resulted in peak estradiol concentrations of 8,321 pg/mL at 5 minutes post-injection.[38] Estradiol levels decreased to 1,628 pg/mL after 30 minutes, to 778 pg/mL after 1 hour, and to 23 pg/mL after 6 hours.[38] Leyendecker et al. (1975) reported that a single intravenous injection of 20 mg estradiol resulted in estradiol levels of 2,950 pg/mL at 12 hours after the injection (earlier time points were not measured).[282] Following this, estradiol levels decreased to around 400 pg/mL by 24 hours post-injection and reached near-baseline levels of 45 pg/mL after 48 hours.[282] The ratio of estradiol to estrone is very high initially (e.g., around 10:1 at peak) but becomes smaller as estradiol levels decline.[38][282] The distribution half-life of intravenous estradiol is about 6 minutes and the terminal half-life of intravenous estradiol is about 1 to 2 hours.[9][11][4] The peak estradiol levels are far higher and the duration far shorter when estradiol is given by intravenous injection than when estradiol esters are administered by intramuscular or subcutaneous injection.[282][9]
The administration of estradiol valerate by intravenous injection has been studied as well.[4][285] It has been found to be very rapidly cleaved into estradiol in the blood.[4][285] The metabolism of estradiol valerate does not differ with intravenous versus intramuscular injection.[285]
While estradiol itself has not been used clinically by intravenous injection, certain estrogen preparations such as conjugated estrogens and estramustine phosphate are available in formulations indicated for intravenous injection.[286] Both of these medications act in part as prodrugs of estradiol.[287][288][289] The intravenous formulation of conjugated estrogens is available at a dose of 25 mg per injection and is used in the treatment of abnormal uterine bleeding due to its ability to rapidly and temporarily enhance coagulation.[286] It has also been used off-label to treat severe bleeding after hysteroscopic metroplasty and as an emergency contraceptive.[286][290][288] The formulation is given in a single injection but can be repeated after 6 to 12 hours if necessary.[286][290][288] Intravenous estramustine phosphate has a relatively long duration and, like oral estramustine phosphate, is used in the treatment of prostate cancer.[289][291] Estramustine phosphate was initially introduced as an intravenous formulation and was only later introduced as an oral medication.[291] Following introduction of the more convenient oral formulation, intravenous estramustine phosphate has largely been abandoned.[291]
The administration of large doses of estrogens intravenously has been studied.[292][293][294]
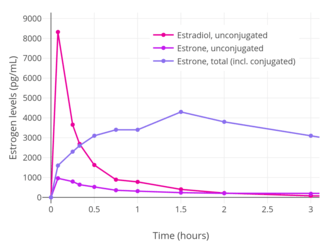 Baseline-corrected estrogen levels after a single intravenous injection of 0.3 mg estradiol in premenopausal women.[38]
Baseline-corrected estrogen levels after a single intravenous injection of 0.3 mg estradiol in premenopausal women.[38]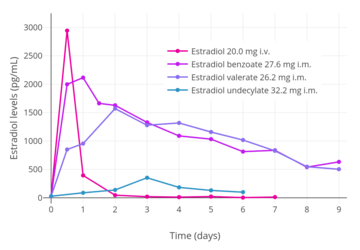 Estradiol levels after equimolar-dose injections of estradiol, estradiol benzoate, estradiol valerate, and estradiol undecylate in women.[282][295] Estradiol was by intravenous injection (i.v.) while the estradiol esters were by intramuscular injection (i.m.).[282][295] Earlier time points than 12 hours post-injection for estradiol i.v. were not measured.[282][295]
Estradiol levels after equimolar-dose injections of estradiol, estradiol benzoate, estradiol valerate, and estradiol undecylate in women.[282][295] Estradiol was by intravenous injection (i.v.) while the estradiol esters were by intramuscular injection (i.m.).[282][295] Earlier time points than 12 hours post-injection for estradiol i.v. were not measured.[282][295]
General
Absorption
Estradiol is well-absorbed regardless of route of administration.[9] However, the bioavailability of estradiol differs substantially with different routes of administration.[9][4] Oral estradiol has an average bioavailability of around 5%, requiring relatively high dosages of estradiol for effects.[9] Estradiol administered in the form of an ester by intramuscular or subcutaneous injection has complete bioavailability.[4][251][8]
Distribution
Estradiol is rapidly distributed throughout the body, with a distribution phase of about 6 minutes following intravenous injection.[11] Due to binding to the ERs, estradiol is preferentially concentrated in tissues with the highest ER content.[11] In animals, these tissues have included the pituitary gland, hypothalamus, other brain regions, uterus, liver, vagina, and adrenals, among other tissues.[11] In contrast to estradiol, likely due to its low affinities for the ERs, estrone is not accumulated in target tissues.[9] Estradiol has been found to cross the blood–brain barrier in rhesus monkeys.[11] The volume of distribution of estradiol has been found to be 0.85 to 1.17 L/kg.[11]
In terms of plasma protein binding, estradiol is bound loosely to albumin and tightly to SHBG, with approximately 97 to 98% of estradiol bound to plasma proteins.[2] In the circulation, approximately 38% of estradiol is bound to SHBG and 60% is bound to albumin, with 2 to 3% free or unbound.[3] However, with oral estradiol, there is an increase in hepatic SHBG production and hence SHBG levels (e.g., +50%), and this results in a relatively reduced fraction of free estradiol.[1][17] As only free estradiol that is not bound to plasma proteins or SHBG is biologically active, this may reduce the potency of oral estradiol by some degree.[11][17] However, a study found that the free fraction of estradiol was similar with doses of oral and topical estradiol that resulted in equivalent total estradiol levels.[81]
Metabolism
There are several major pathways of estradiol metabolism, which occur both in the liver and in other tissues:[11][9][1]
- Dehydrogenation by 17β-hydroxysteroid dehydrogenase (17β-HSD) into estrone
- Conjugation by estrogen sulfotransferases and UDP-glucuronyltransferases into C3 and/or C17β estrogen conjugates like estrone sulfate and estradiol glucuronide
- Hydroxylation by cytochrome P450 enzymes such as CYP1A1 and CYP3A4 into catechol estrogens like 2-hydroxyestrone and 2-hydroxyestradiol as well as 16-hydroxylated estrogens like 16α-hydroxyestrone and estriol (16α-hydroxyestradiol)
Both dehydrogenation of estradiol by 17β-HSD into estrone and conjugation into estrogen conjugates are reversible transformations.[11][9] However, in regards to sulfation and desulfation, transformation of estrone into estrone sulfate is predominant relative to the reverse reaction.[11][62]
Estradiol can also be reversibly converted into long-lived lipoidal estradiol forms like estradiol palmitate and estradiol stearate as a minor route of metabolism.[10]
The elimination half-life of estradiol administered via intravenous injection is 2 hours in men and 50 minutes in women.[4] Other routes of administration of estradiol like oral administration or intramuscular injection have far longer elimination half-lives and durations of action due to (1) the formation of a large circulating reservoir of metabolism-resistant estrogen conjugates that can be reconverted back into estradiol and/or (2) the formation of slowly-releasing depots.[11][9]
The metabolic clearance rates of estradiol, estrone, and estrone sulfate are 580 L/day/m2, 1,050 L/day/m2, and 80 L/day/m2, respectively.[9]
Elimination
A single dose of oral estradiol valerate is eliminated 54% in urine and 6% in feces.[1] A substantial amount of estradiol is also excreted in bile.[1] The urinary metabolites of estradiol are predominantly present in the form of estrogen conjugates, including glucuronides and, to a lesser extent, sulfates.[1] The main metabolites of estradiol in urine are estrone glucuronide (13–30%), 2-hydroxyestrone (2.6–10.1%), unchanged estradiol (5.2–7.5%), estriol (2.0–5.9%), and 16α-hydroxyestrone (1.0–2.9%).[1]
See also
References
- Stanczyk, Frank Z.; Archer, David F.; Bhavnani, Bhagu R. (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–727. doi:10.1016/j.contraception.2012.12.011. ISSN 0010-7824. PMID 23375353.
- O'Connell MB (1995). "Pharmacokinetic and pharmacologic variation between different estrogen products". J Clin Pharmacol. 35 (9 Suppl): 18S–24S. doi:10.1002/j.1552-4604.1995.tb04143.x. PMID 8530713.
- Kuhnz, W.; Blode, H.; Zimmermann, H. (1993). "Pharmacokinetics of Exogenous Natural and Synthetic Estrogens and Antiestrogens". Estrogens and Antiestrogens II. Handbook of Experimental Pharmacology. 135 / 2. pp. 261–322. doi:10.1007/978-3-642-60107-1_15. ISBN 978-3-642-64261-6. ISSN 0171-2004.
- Düsterberg B, Nishino Y (1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. doi:10.1016/0378-5122(82)90064-0. PMID 7169965.
- Tommaso Falcone; William W. Hurd (2007). Clinical Reproductive Medicine and Surgery. Elsevier Health Sciences. pp. 22, 362, 388. ISBN 978-0-323-03309-1.
- Price, T; Blauer, K; Hansen, M; Stanczyk, F; Lobo, R; Bates, G (1997). "Single-dose pharmacokinetics of sublingual versus oral administration of micronized 17-estradiol". Obstetrics & Gynecology. 89 (3): 340–345. doi:10.1016/S0029-7844(96)00513-3. ISSN 0029-7844. PMID 9052581.
- Naunton, Mark; Al Hadithy, Asmar F. Y.; Brouwers, Jacobus R. B. J.; Archer, David F. (2006). "Estradiol gel". Menopause. 13 (3): 517–527. doi:10.1097/01.gme.0000191881.52175.8c. ISSN 1072-3714. PMID 16735950.
- Sierra-Ramírez JA, Lara-Ricalde R, Lujan M, Velázquez-Ramírez N, Godínez-Victoria M, Hernádez-Munguía IA, Padilla A, Garza-Flores J (2011). "Comparative pharmacokinetics and pharmacodynamics after subcutaneous and intramuscular administration of medroxyprogesterone acetate (25 mg) and estradiol cypionate (5 mg)". Contraception. 84 (6): 565–70. doi:10.1016/j.contraception.2011.03.014. PMID 22078184.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 121, 226, 235–237. ISBN 978-3-642-58616-3.
- Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 163–178, 235–237, 252–253, 261–276, 538–543. ISBN 978-3-642-60107-1.
- Stege R, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A (1988). "Single drug polyestradiol phosphate therapy in prostatic cancer". Am. J. Clin. Oncol. 11 Suppl 2: S101–3. doi:10.1097/00000421-198801102-00024. PMID 3242384.
- Ockrim JL, Lalani EN, Laniado ME, Carter SS, Abel PD (May 2003). "Transdermal estradiol therapy for advanced prostate cancer--forward to the past?". J. Urol. 169 (5): 1735–7. doi:10.1097/01.ju.0000061024.75334.40. PMID 12686820.
- http://www.ilexmedical.com/files/PDF/Estradiol_ARC.pdf
- Rogerio A. Lobo (5 June 2007). Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Academic Press. pp. 177, 217–226, 770–771. ISBN 978-0-08-055309-2.
- M. Notelovitz; P.A. van Keep (6 December 2012). The Climacteric in Perspective: Proceedings of the Fourth International Congress on the Menopause, held at Lake Buena Vista, Florida, October 28 – November 2, 1984. Springer Science & Business Media. pp. 397, 399. ISBN 978-94-009-4145-8.
[...] following the menopause, circulating estradiol levels decrease from a premenopausal mean of 120 pg/ml to only 13 pg/ml.
- C. Christian; B. von Schoultz (15 March 1994). Hormone Replacement Therapy: Standardized or Individually Adapted Doses?. CRC Press. pp. 9–16, 60. ISBN 978-1-85070-545-1.
The mean integrated estradiol level during a full 28-day normal cycle is around 80 pg/ml.
- Eugenio E. Müller; Robert M. MacLeod (6 December 2012). Neuroendocrine Perspectives. Springer Science & Business Media. pp. 121–. ISBN 978-1-4612-3554-5.
[...] [premenopausal] mean [estradiol] concentration of 150 pg/ml [...]
- Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 889, 1059–1060, 2153. ISBN 978-0-7817-1750-2.
- Troisi R, Potischman N, Roberts JM, Harger G, Markovic N, Cole B, Lykins D, Siiteri P, Hoover RN (2003). "Correlation of serum hormone concentrations in maternal and umbilical cord samples". Cancer Epidemiol. Biomarkers Prev. 12 (5): 452–6. PMID 12750241.
- A. Wayne Meikle (1 June 1999). Hormone Replacement Therapy. Springer Science & Business Media. pp. 380–. ISBN 978-1-59259-700-0.
- V. H. T. James; J. R. Pasqualini (22 October 2013). Hormonal Steroids: Proceedings of the Sixth International Congress on Hormonal Steroids. Elsevier Science. pp. 821–. ISBN 978-1-4831-9067-9.
- Dosage Form Design Considerations. Elsevier Science. 28 July 2018. pp. 159–. ISBN 978-0-12-814424-4.
- Frank Z. Stanczyk (1998). "Pharmacological background of estrogen replacement therapy and continuance". In Ian S. Fraser; R. P. S. Jansen; R. A. Lobo; M. I. Whitehead (eds.). Estrogens and Progestogens in Clinical Practice. Churchill Livingstone. pp. 655–666. ISBN 978-0-443-04706-0.
- Yen, S. S. C.; Martin, P. L.; Burnier, A. M.; Czekala, N. M.; Greaney, M. O.; Callantine, M. R. (1975). "Circulating Estradiol, Estrone and Gonadotropin Levels Following the Administration of Orally Active 17β-Estradiol in Postmenopausal Women". The Journal of Clinical Endocrinology & Metabolism. 40 (3): 518–521. doi:10.1210/jcem-40-3-518. ISSN 0021-972X. PMID 1117058.
- Devroey P, Pados G (1998). "Preparation of endometrium for egg donation". Hum. Reprod. Update. 4 (6): 856–61. doi:10.1093/humupd/4.6.856. PMID 10098476.
Oestradiol valerate and oestradiol in a micronized form are the most widely used oestrogen per os for steroid substitution therapy. Our regimen, as of most other groups [...] is oestradiol valerate (Progynova; Schering, Berlin, Germany) given in various concentrations throughout the cycle [...]. According to Norfolk's protocol, 2 mg of micronized oestradiol valerate are given on cycle days 1–5. [...] In tablet form, micronized oestradiol valerate is also efficiently absorbed [...]
- Martin PL, Burnier AM, Greaney MO (1972). "Oral menopausal therapy using 17- micronized estradiol. A preliminary study of effectiveness, tolerance and patient preference". Obstet Gynecol. 39 (5): 771–4. doi:10.1097/00006250-197205000-00022 (inactive 2 December 2019). PMID 5023261.
- Herr, F.; Revesz, C.; Manson, A. J.; Jewell, J. B. (1970). "Biological Properties of Estrogen Sulfates". Chemical and Biological Aspects of Steroid Conjugation. pp. 368–408. doi:10.1007/978-3-642-49793-3_8. ISBN 978-3-642-49506-9.
- Jorge Martinez-Manautou; Harry W. Rudel (1966). "Antiovulatory Activity of Several Synthetic and Natural Estrogens". In Robert Benjamin Greenblatt (ed.). Ovulation: Stimulation, Suppression, and Detection. Lippincott. pp. 243–253.
- J. Horsky; J. Presl (6 December 2012). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 313–. ISBN 978-94-009-8195-9.
- Janet Brotherton (1976). Sex Hormone Pharmacology. Academic Press. p. 34. ISBN 978-0-12-137250-7.
- Gibian H, Kopp R, Kramer M, Neumann F, Richter H (1968). "Effect of particle size on biological activity of norethisterone acetate". Acta Physiol Lat Am. 18 (4): 323–6. PMID 5753386.
- He CH, Shi YE, Liao DL, Zhu YH, Xu JQ, Matlin SA, Vince PM, Fotherby K, Van Look PF (May 1990). "Comparative cross-over pharmacokinetic study on two types of postcoital contraceptive tablets containing levonorgestrel". Contraception. 41 (5): 557–67. doi:10.1016/0010-7824(90)90064-3. PMID 2112080.
- Gunther Göretzlehner; Christian Lauritzen; Thomas Römer (30 November 2011). Praktische Hormontherapie in der Gynäkologie. Walter de Gruyter. pp. 44–. ISBN 978-3-11-024568-4. Unknown parameter
|coauthors=ignored (|author=suggested) (help) - Rydén, Ake B. V. (1950). "Natural and synthetic oestrogenic substances: their relative effectiveness when administered orally". Acta Endocrinologica. 4 (2): 121–139. doi:10.1530/acta.0.0040121. ISSN 0804-4643. PMID 15432047.
- Rydén, Åke (1947). "Natural and synthetic estrogenic substances – A comparison of the effect upon the endometrium in castrated women". Acta Pathologica Microbiologica Scandinavica. 24 (3–4): 213–241. doi:10.1111/j.1699-0463.1947.tb00592.x. ISSN 0365-5555.
- Kottmeier, Hans Ludvig (1947). "Ueber blutungen in der menopause: Speziell der klinischen bedeutung eines endometriums mit zeichen hormonaler beeinflussung: Part I". Acta Obstetricia et Gynecologica Scandinavica. 27 (s6): 1–121. doi:10.3109/00016344709154486. ISSN 0001-6349.
There is no doubt that the conversion of the endometrium with injections of both synthetic and native estrogenic hormone preparations succeeds, but the opinion whether native, orally administered preparations can produce a proliferation mucosa changes with different authors. PEDERSEN-BJERGAARD (1939) was able to show that 90% of the folliculin taken up in the blood of the vena portae is inactivated in the liver. Neither KAUFMANN (1933, 1935), RAUSCHER (1939, 1942) nor HERRNBERGER (1941) succeeded in bringing a castration endometrium into proliferation using large doses of orally administered preparations of estrone or estradiol. Other results are reported by NEUSTAEDTER (1939), LAUTERWEIN (1940) and FERIN (1941); they succeeded in converting an atrophic castration endometrium into an unambiguous proliferation mucosa with 120–300 mg oestradiol or with 380 mg oestrone.
- Kuhnz W, Gansau C, Mahler M (September 1993). "Pharmacokinetics of estradiol, free and total estrone, in young women following single intravenous and oral administration of 17β-estradiol". Arzneimittelforschung. 43 (9): 966–73. ISSN 0004-4172. PMID 8240460.
- Leinung MC, Feustel PJ, Joseph J (2018). "Hormonal Treatment of Transgender Women with Oral Estradiol". Transgend Health. 3 (1): 74–81. doi:10.1089/trgh.2017.0035. PMC 5944393. PMID 29756046.
- Shellenberger, T. E. (1986). "Pharmacology of estrogens". The Climacteric in Perspective. pp. 393–410. doi:10.1007/978-94-009-4145-8_36. ISBN 978-94-010-8339-3.
Ethinyl estradiol is a synthetic and comparatively potent estrogen. As a result of the alkylation in 17-C position it is not a substrate for 17β dehydrogenase, an enzyme which transforms natural estradiol-17β to the less potent estrone in target organs.
- Ellis MJ, Gao F, Dehdashti F, Jeffe DB, Marcom PK, Carey LA, Dickler MN, Silverman P, Fleming GF, Kommareddy A, Jamalabadi-Majidi S, Crowder R, Siegel BA (August 2009). "Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study". JAMA. 302 (7): 774–80. doi:10.1001/jama.2009.1204. PMC 3460383. PMID 19690310.
- Selby P, McGarrigle HH, Peacock M (March 1989). "Comparison of the effects of oral and transdermal oestradiol administration on oestrogen metabolism, protein synthesis, gonadotrophin release, bone turnover and climacteric symptoms in postmenopausal women". Clin. Endocrinol. (Oxf). 30 (3): 241–9. doi:10.1111/j.1365-2265.1989.tb02232.x. PMID 2512035.
- Rebekah Wang-Cheng; Joan M. Neuner; Vanessa M. Barnabei (2007). Menopause. ACP Press. pp. 91–. ISBN 978-1-930513-83-9.
- John J.B. Anderson; Sanford C. Garner (24 October 1995). Calcium and Phosphorus in Health and Disease. CRC Press. pp. 215–216. ISBN 978-0-8493-7845-4.
- Slater CC, Hodis HN, Mack WJ, Shoupe D, Paulson RJ, Stanczyk FZ (2001). "Markedly elevated levels of estrone sulfate after long-term oral, but not transdermal, administration of estradiol in postmenopausal women". Menopause. 8 (3): 200–3. doi:10.1097/00042192-200105000-00009. PMID 11355042.
- Eef Hogervorst (24 September 2009). Hormones, Cognition and Dementia: State of the Art and Emergent Therapeutic Strategies. Cambridge University Press. pp. 82–. ISBN 978-0-521-89937-6.
- Wright JV (December 2005). "Bio-identical steroid hormone replacement: selected observations from 23 years of clinical and laboratory practice". Ann. N. Y. Acad. Sci. 1057 (1): 506–24. Bibcode:2005NYASA1057..506W. doi:10.1196/annals.1356.039. PMID 16399916.
- Friel PN, Hinchcliffe C, Wright JV (March 2005). "Hormone replacement with estradiol: conventional oral doses result in excessive exposure to estrone". Altern Med Rev. 10 (1): 36–41. PMID 15771561.
- Lobo RA (March 1987). "Absorption and metabolic effects of different types of estrogens and progestogens". Obstet. Gynecol. Clin. North Am. 14 (1): 143–67. PMID 3306517.
- Potts RO, Lobo RA (May 2005). "Transdermal drug delivery: clinical considerations for the obstetrician-gynecologist". Obstet Gynecol. 105 (5 Pt 1): 953–61. doi:10.1097/01.AOG.0000161958.70059.db. PMID 15863530.
- Laura Marie Borgelt (2010). Women's Health Across the Lifespan: A Pharmacotherapeutic Approach. ASHP. pp. 257–. ISBN 978-1-58528-194-7.
- Jose Russo; Irma H. Russo (28 June 2011). Molecular Basis of Breast Cancer: Prevention and Treatment. Springer Science & Business Media. pp. 92–. ISBN 978-3-642-18736-0.
- Sue E. Huether; Kathryn L. McCance (27 December 2013). Understanding Pathophysiology. Elsevier Health Sciences. pp. 845–. ISBN 978-0-323-29343-3.
- Ruggiero RJ, Likis FE (2002). "Estrogen: physiology, pharmacology, and formulations for replacement therapy". Journal of Midwifery & Women's Health. 47 (3): 130–8. doi:10.1016/s1526-9523(02)00233-7. PMID 12071379.
- Escande A, Pillon A, Servant N, Cravedi JP, Larrea F, Muhn P, Nicolas JC, Cavaillès V, Balaguer P (2006). "Evaluation of ligand selectivity using reporter cell lines stably expressing estrogen receptor alpha or beta". Biochem. Pharmacol. 71 (10): 1459–69. doi:10.1016/j.bcp.2006.02.002. PMID 16554039.
- Powers MS, Schenkel L, Darley PE, Good WR, Balestra JC, Place VA (August 1985). "Pharmacokinetics and pharmacodynamics of transdermal dosage forms of 17 beta-estradiol: comparison with conventional oral estrogens used for hormone replacement". Am. J. Obstet. Gynecol. 152 (8): 1099–106. doi:10.1016/0002-9378(85)90569-1. PMID 2992279.
- Fåhraeus L, Larsson-Cohn U (December 1982). "Oestrogens, gonadotrophins and SHBG during oral and cutaneous administration of oestradiol-17 beta to menopausal women". Acta Endocrinol. 101 (4): 592–6. doi:10.1530/acta.0.1010592. PMID 6818806.
- Kloosterboer, HJ; Schoonen, WG; Verheul, HA (11 April 2008). "Proliferation of Breast Cells by Steroid Hormones and Their Metabolites". In Pasqualini, Jorge R (ed.). Breast Cancer: Prognosis, Treatment, and Prevention. CRC Press. pp. 343–366. ISBN 978-1-4200-5873-4.
- Sasson S, Notides AC (July 1983). "Estriol and estrone interaction with the estrogen receptor. II. Estriol and estrone-induced inhibition of the cooperative binding of [3H]estradiol to the estrogen receptor". J. Biol. Chem. 258 (13): 8118–22. PMID 6863280.
- Lundström E, Conner P, Naessén S, Löfgren L, Carlström K, Söderqvist G (2015). "Estrone - a partial estradiol antagonist in the normal breast". Gynecol. Endocrinol. 31 (9): 747–9. doi:10.3109/09513590.2015.1062866. PMID 26190536.
- Martel C, Rhéaume E, Takahashi M, Trudel C, Couët J, Luu-The V, Simard J, Labrie F (March 1992). "Distribution of 17 beta-hydroxysteroid dehydrogenase gene expression and activity in rat and human tissues". J. Steroid Biochem. Mol. Biol. 41 (3–8): 597–603. doi:10.1016/0960-0760(92)90390-5. PMID 1314080.
- Miki Y, Nakata T, Suzuki T, Darnel AD, Moriya T, Kaneko C, Hidaka K, Shiotsu Y, Kusaka H, Sasano H (December 2002). "Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues". J. Clin. Endocrinol. Metab. 87 (12): 5760–8. doi:10.1210/jc.2002-020670. PMID 12466383.
- Quirk, J. Gerald; Wendel, George D. (1983). "Biologic Effects of Natural and Synthetic Estrogens". In J.J. Buchsbaum (ed.). The Menopause. pp. 55–75. doi:10.1007/978-1-4612-5525-3_5. ISBN 978-1-4612-5525-3. ISSN 0178-0328.
- Laura Marie Borgelt (2010). Women's Health Across the Lifespan: A Pharmacotherapeutic Approach. ASHP. pp. 256–. ISBN 978-1-58528-194-7.
- Rezvanpour A, Don-Wauchope AC (March 2017). "Clinical implications of estrone sulfate measurement in laboratory medicine". Crit Rev Clin Lab Sci. 54 (2): 73–86. doi:10.1080/10408363.2016.1252310. PMID 27960570.
- Crandall CJ, Guan M, Laughlin GA, Ursin GA, Stanczyk FZ, Ingles SA, Barrett-Connor E, Greendale GA (July 2008). "Increases in serum estrone sulfate level are associated with increased mammographic density during menopausal hormone therapy". Cancer Epidemiol. Biomarkers Prev. 17 (7): 1674–81. doi:10.1158/1055-9965.EPI-07-2779. PMC 2745228. PMID 18628419.
- Jorge R. Pasqualini (17 July 2002). Breast Cancer: Prognosis, Treatment, and Prevention. CRC Press. pp. 195–. ISBN 978-0-203-90924-9.
- James MR, Skaar TC, Lee RY, MacPherson A, Zwiebel JA, Ahluwalia BS, Ampy F, Clarke R (April 2001). "Constitutive expression of the steroid sulfatase gene supports the growth of MCF-7 human breast cancer cells in vitro and in vivo". Endocrinology. 142 (4): 1497–505. doi:10.1210/endo.142.4.8091. PMID 11250930.
- Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 753–. ISBN 978-1-4511-4847-3.
- Alkjaersig N, Fletcher AP, de Ziegler D, Steingold KA, Meldrum DR, Judd HL (1988). "Blood coagulation in postmenopausal women given estrogen treatment: comparison of transdermal and oral administration". J. Lab. Clin. Med. 111 (2): 224–8. PMID 2448408.
- Nugent AG, Leung KC, Sullivan D, Reutens AT, Ho KK (2003). "Modulation by progestogens of the effects of oestrogen on hepatic endocrine function in postmenopausal women". Clin. Endocrinol. 59 (6): 690–8. doi:10.1046/j.1365-2265.2003.01907.x. PMID 14974909.
- Isotton, A. L.; Wender, M. C. O.; Casagrande, A.; Rollin, G.; Czepielewski, M. A. (2011). "Effects of oral and transdermal estrogen on IGF1, IGFBP3, IGFBP1, serum lipids, and glucose in patients with hypopituitarism during GH treatment: a randomized study" (PDF). European Journal of Endocrinology. 166 (2): 207–213. doi:10.1530/EJE-11-0560. ISSN 0804-4643. PMID 22108915.
- Jasonni VM, Bulletti C, Naldi S, Ciotti P, Di Cosmo D, Lazzaretto R, Flamigni C (1988). "Biological and endocrine aspects of transdermal 17 beta-oestradiol administration in post-menopausal women". Maturitas. 10 (4): 263–70. doi:10.1016/0378-5122(88)90062-x. PMID 3226336.
- Weissberger AJ, Ho KK, Lazarus L (1991). "Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women". J. Clin. Endocrinol. Metab. 72 (2): 374–81. doi:10.1210/jcem-72-2-374. PMID 1991807.
- Sonnet E, Lacut K, Roudaut N, Mottier D, Kerlan V, Oger E (2007). "Effects of the route of oestrogen administration on IGF-1 and IGFBP-3 in healthy postmenopausal women: results from a randomized placebo-controlled study". Clin. Endocrinol. 66 (5): 626–31. doi:10.1111/j.1365-2265.2007.02783.x. PMID 17492948.
- Dansuk R, Unal O, Karageyim Y, Esim E, Turan C (2004). "Evaluation of the effect of tibolone and transdermal estradiol on triglyceride level in hypertriglyceridemic and normotriglyceridemic postmenopausal women". Gynecol. Endocrinol. 18 (5): 233–9. doi:10.1080/09513590410001715199. PMID 15346658.
- Notelovitz M (March 2006). "Clinical opinion: the biologic and pharmacologic principles of estrogen therapy for symptomatic menopause". MedGenMed. 8 (1): 85. PMC 1682006. PMID 16915215.
- Goodman MP (February 2012). "Are all estrogens created equal? A review of oral vs. transdermal therapy". J Womens Health (Larchmt). 21 (2): 161–9. doi:10.1089/jwh.2011.2839. PMID 22011208.
- Nachtigall LE, Raju U, Banerjee S, Wan L, Levitz M (2000). "Serum estradiol-binding profiles in postmenopausal women undergoing three common estrogen replacement therapies: associations with sex hormone-binding globulin, estradiol, and estrone levels". Menopause. 7 (4): 243–50. doi:10.1097/00042192-200007040-00006. ISSN 1072-3714. PMID 10914617.
- Santoro N, Worsley R, Miller KK, Parish SJ, Davis SR (March 2016). "Role of Estrogens and Estrogen-Like Compounds in Female Sexual Function and Dysfunction". J Sex Med. 13 (3): 305–16. doi:10.1016/j.jsxm.2015.11.015. PMID 26944462.
- Nilsson B, Holst J, von Schoultz B (October 1984). "Serum levels of unbound 17 beta-oestradiol during oral and percutaneous postmenopausal replacement therapy". Br J Obstet Gynaecol. 91 (10): 1031–6. doi:10.1111/j.1471-0528.1984.tb03683.x. PMID 6541503.
- Kicovic PM, Luisi M, Franchi F, Alicicco E (July 1977). "Effects of orally administered oestradiol decanoate on plasma oestradiol, oestrone and gonadotrophin levels, vaginal cytology, cervical mucus and endometrium in ovariectomized women". Clin. Endocrinol. (Oxf). 7 (1): 73–7. doi:10.1111/j.1365-2265.1977.tb02941.x. PMID 880735.
- Luisi M, Kicovic PM, Alicicco E, Franchi F (1978). "Effects of estradiol decanoate in ovariectomized women". J. Endocrinol. Invest. 1 (2): 101–6. doi:10.1007/BF03350355. PMID 755846.
- de Visser J, van der Vies J (June 1977). "Oestrogenic activity of oestradiol-decanoate after oral administration to rodents". Acta Endocrinol. 85 (2): 422–8. doi:10.1530/acta.0.0850422. PMID 577331.
- Ranjit Roy Chaudhury (1 January 1981). Pharmacology of Estrogens. Elsevier Science & Technology Books. p. 36. ISBN 978-0-08-026869-9.
- Bastianelli, Carlo; Farris, Manuela; Rosato, Elena; Brosens, Ivo; Benagiano, Giuseppe (2018). "Pharmacodynamics of combined estrogen-progestin oral contraceptives 3. Inhibition of ovulation". Expert Review of Clinical Pharmacology. 11 (11): 1085–1098. doi:10.1080/17512433.2018.1536544. ISSN 1751-2433.
- Dahlgren E, Crona N, Janson PO, Samsioe G (1985). "Oral replacement with estradiol-cyclooctyl acetate: a new estradiol analogue. Effects on serum lipids, proteins, gonadotrophins, estrogens and uterine endometrial morphology". Gynecol. Obstet. Invest. 20 (2): 84–90. doi:10.1159/000298978. PMID 3932144.
- Schubert W, Cullberg G (1987). "Ovulation inhibition with 17 beta-estradiol cyclo-octyl acetate and desogestrel". Acta Obstet Gynecol Scand. 66 (6): 543–7. doi:10.3109/00016348709015732. PMID 2962418.
- Schubert W, Cullberg G (1988). "Fat-soluble 17 beta-estradiol: a way of reducing dosage in steroid hormonal substitution?". Acta Obstet Gynecol Scand. 67 (3): 271–5. doi:10.3109/00016348809004218. PMID 2972162.
- Elger W, Wyrwa R, Ahmed G, Meece F, Nair HB, Santhamma B, Killeen Z, Schneider B, Meister R, Schubert H, Nickisch K (January 2017). "Estradiol prodrugs (EP) for efficient oral estrogen treatment and abolished effects on estrogen modulated liver functions". J. Steroid Biochem. Mol. Biol. 165 (Pt B): 305–311. doi:10.1016/j.jsbmb.2016.07.008. PMID 27449818.
- Ahmed G, Elger W, Meece F, Nair HB, Schneider B, Wyrwa R, Nickisch K (October 2017). "A prodrug design for improved oral absorption and reduced hepatic interaction". Bioorg. Med. Chem. 25 (20): 5569–5575. doi:10.1016/j.bmc.2017.08.027. PMID 28886996.
- Pickar JH, Bon C, Amadio JM, Mirkin S, Bernick B (December 2015). "Pharmacokinetics of the first combination 17β-estradiol/progesterone capsule in clinical development for menopausal hormone therapy". Menopause. 22 (12): 1308–16. doi:10.1097/GME.0000000000000467. PMC 4666011. PMID 25944519.
- Yen SS, Martin PL, Burnier AM, Czekala NM, Greaney MO, Callantine MR (March 1975). "Circulating estradiol, estrone and gonadotropin levels following the administration of orally active 17beta-estradiol in postmenopausal women". J. Clin. Endocrinol. Metab. 40 (3): 518–21. doi:10.1210/jcem-40-3-518. PMID 1117058.
- Center for Drug Evaluation and Research. Office of Generic Drugs. Division of Bioequivalence. Application Number: 40326. Review of a Fasting Bioequivalence Study, Dissolution Data and Two Waiver Requests. Estradiol Tablets. ANDA # 40-326. Mylan Pharmaceuticals. https://www.accessdata.fda.gov/drugsatfda_docs/anda/99/40326_estradiol_bioeqr.pdf
- Wiegratz I, Fink T, Rohr UD, Lang E, Leukel P, Kuhl H (September 2001). "Überkreuz-Vergleich der Pharmakokinetik von Estradiol unter der Hormonsubstitution mit Estradiolvalerat oder mikronisiertem Estradiol" [Cross-over comparison of the pharmacokinetics of estradiol during hormone replacement therapy with estradiol valerate or micronized estradiol]. Zentralbl Gynakol (in German). 123 (9): 505–12. doi:10.1055/s-2001-18223. PMID 11709743.
- Wren BG, Day RO, McLachlan AJ, Williams KM (June 2003). "Pharmacokinetics of estradiol, progesterone, testosterone and dehydroepiandrosterone after transbuccal administration to postmenopausal women". Climacteric. 6 (2): 104–11. doi:10.1080/cmt.6.2.104.111. PMID 12841880.
- Göretzlehner, G (1990). "Wirkspektrum unterschiedlicher Östrogene" [Spectrum of different estrogens]. In Wolf, Alfred S.; Schneider, H.P.G. (eds.). Östrogene in Diagnostik und Therapie [Estrogens in diagnostics and therapy] (in German). Springer-Verlag. pp. 77–83. doi:10.1007/978-3-642-75101-1_9. ISBN 978-3-642-75101-1.
- Gass MS, Rebar RW, Cuffie-Jackson C, Cedars MI, Lobo RA, Shoupe D, Judd HL, Buyalos RP, Clisham PR (October 2004). "A short study in the treatment of hot flashes with buccal administration of 17-beta estradiol". Maturitas. 49 (2): 140–7. doi:10.1016/j.maturitas.2003.12.004. PMID 15474758.
- Chandrasekhara, Darshna Somaiya; Ali, Vaseem; Prost, Henry Michael; Nader-Estekhari, Shahla (2002). "Buccal estrogen in toothpaste study: systemic absorption of estradiol in postmenopausal or surgically menopausal women when administered as a component in toothpaste". Fertility and Sterility. 78: S98. doi:10.1016/S0015-0282(02)03639-7. ISSN 0015-0282.
- Perloff WH (January 1950). "Estradiol buccal tablets in the treatment of the menopause". Am. J. Obstet. Gynecol. 59 (1): 223–5. doi:10.1016/0002-9378(50)90390-5. PMID 15408716.
- Hall GJ (April 1949). "The buccal administration of estradiol". J. Clin. Endocrinol. Metab. 9 (4): 382–4. doi:10.1210/jcem-9-4-382. PMID 18120722.
- Bodor, Nicholas; Buchwald, Peter (2006). "Brain-Targeted Delivery of Estradiol". American Journal of Drug Delivery. 4 (3): 161–175. doi:10.2165/00137696-200604030-00004. ISSN 1175-9038.
- van der Bijl P, van Eyk AD, Thompson IO (April 1998). "Permeation of 17beta-estradiol through human vaginal and buccal mucosa". Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 85 (4): 393–8. doi:10.1016/S1079-2104(98)90063-4. PMID 9574947.
- Nicolazzo JA, Reed BL, Finnin BC (February 2004). "Assessment of the effects of sodium dodecyl sulfate on the buccal permeability of caffeine and estradiol". J Pharm Sci. 93 (2): 431–40. doi:10.1002/jps.10559. PMID 14705199.
- Kitano, Manabu; Maitani, Yoshie; Takayama, Kozo; Nagai, Tsuneji (1998). "Buccal absorption through golden hamster cheek pouch in vitro and in vivo of 17β-estradiol from hydrogels containing three types of absorption enhancers". International Journal of Pharmaceutics. 174 (1–2): 19–28. doi:10.1016/S0378-5173(98)00234-8. ISSN 0378-5173.
- Nicolazzo JA, Reed BL, Finnin BC (April 2005). "Enhanced buccal mucosal retention and reduced buccal permeability of estradiol in the presence of padimate O and Azone: a mechanistic study". J Pharm Sci. 94 (4): 873–82. doi:10.1002/jps.20240. PMID 15736191.
- Pines A, Averbuch M, Fisman EZ, Rosano GM (September 1999). "The acute effects of sublingual 17beta-estradiol on the cardiovascular system". Maturitas. 33 (1): 81–5. doi:10.1016/S0378-5122(99)00036-5. PMID 10585176.
- Casper RF, Yen SS (January 1981). "Rapid absorption of micronized estradiol-17 beta following sublingual administration". Obstet Gynecol. 57 (1): 62–4. PMID 7454177.
- General Practitioner. American Academy of General Practice. April 1954. p. 168–170.
Diogynets* [...] * brand of estradiol transmucosal tablets, scored: 0.125 mg., 0.25 mg. and 1.0 mg., bottles of 50 and 100.
- Ashton Leroy Welsh (1951). Dermatological Formulary: A Guide for Medical Students and Resident Physicians in Dermatology. Educational Publishers. p. 155.
- University of California (1868-1952) (1952). Hospital Formulary and Compendium of Useful Information. University of California Press. pp. 49–. GGKEY:2UAAZRZ5LN0.
- Allan William Spence (1953). Clinical Endocrinology. Cassell. p. 547.
- Price TM, Blauer KL, Hansen M, Stanczyk F, Lobo R, Bates GW (March 1997). "Single-dose pharmacokinetics of sublingual versus oral administration of micronized 17 beta-estradiol". Obstet Gynecol. 89 (3): 340–5. doi:10.1016/S0029-7844(96)00513-3. PMID 9052581.
- Pines A, Fisman EZ, Drory Y, Shapira I, Averbuch M, Eckstein N, Motro M, Levo Y, Ayalon D (1998). "The effects of sublingual estradiol on left ventricular function at rest and exercise in postmenopausal women: an echocardiographic assessment". Menopause. 5 (2): 79–85. doi:10.1097/00042192-199822010-00004. PMID 9689200.
- Kuhl H (2000). "Pharmacology of estradiol and estriol". Menopause Review. Société Européenne de Ménopause. 5: 23–44. ISSN 1272-9868. OCLC 473540298.
- Fisman EZ, Tenenbaum A, Shapira I, Motro M, Pines A (October 1999). "The acute effects of sublingual estradiol on left ventricular diastolic function in normotensive and hypertensive postmenopausal women". Maturitas. 33 (2): 145–52. doi:10.1016/S0378-5122(99)00051-1. PMID 10597879.
- Ahokas A, Kaukoranta J, Wahlbeck K, Aito M (May 2001). "Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study". J Clin Psychiatry. 62 (5): 332–6. doi:10.4088/JCP.v62n0504. PMID 11411813.
- Ahokas A, Kaukoranta J, Aito M (September 1999). "Effect of oestradiol on postpartum depression". Psychopharmacology. 146 (1): 108–10. doi:10.1007/s002130051095. PMID 10485972.
- Ng RC, Hirata CK, Yeung W, Haller E, Finley PR (September 2010). "Pharmacologic treatment for postpartum depression: a systematic review". Pharmacotherapy. 30 (9): 928–41. doi:10.1592/phco.30.9.928. PMID 20795848.
- di Scalea TL, Wisner KL (November 2009). "Pharmacotherapy of postpartum depression". Expert Opin Pharmacother. 10 (16): 2593–607. doi:10.1517/14656560903277202. PMC 2929691. PMID 19874247.
- Serhal PF, Craft IL (May 1989). "Oocyte donation in 61 patients". Lancet. 1 (8648): 1185–7. doi:10.1016/S0140-6736(89)92762-1. PMID 2566746.
- Serhal P (July 1990). "Oocyte donation and surrogacy". Br. Med. Bull. 46 (3): 796–812. doi:10.1093/oxfordjournals.bmb.a072432. PMID 2207608.
- Lim HH, Jang YH, Choi GY, Lee JJ, Lee ES (January 2019). "Gender affirmative care of transgender people: a single center's experience in Korea". Obstet Gynecol Sci. 62 (1): 46–55. doi:10.5468/ogs.2019.62.1.46. PMC 6333764. PMID 30671393.
When we prescribed estradiol, we preferred sublingual estradiol valerate instead of the oral form for feminizing HT since prior researchers have reported the effectiveness of sublingual administration in maintaining high blood estradiol concentration and low E1/E2 ratio [13].
- Pitha J, Harman SM, Michel ME (February 1986). "Hydrophilic cyclodextrin derivatives enable effective oral administration of steroidal hormones". J Pharm Sci. 75 (2): 165–7. doi:10.1002/jps.2600750213. PMID 3958926.
- Hoon TJ, Dawood MY, Khan-Dawood FS, Ramos J, Batenhorst RL (November 1993). "Bioequivalence of a 17 beta-estradiol hydroxypropyl-beta-cyclodextrin complex in postmenopausal women". J Clin Pharmacol. 33 (11): 1116–21. doi:10.1002/j.1552-4604.1993.tb01949.x. PMID 8300895.
- Fridriksdóttir H, Loftsson T, Gudmundsson JA, Bjarnason GJ, Kjeld M, Thorsteinsson T (January 1996). "Design and in vivo testing of 17 beta-estradiol-HP beta CD sublingual tablets". Pharmazie. 51 (1): 39–42. PMID 8999433.
- Brewster, M. E.; Howes, J.; Griffith, W.; Garty, N.; Bodor, N.; Anderson, W. R.; Pop, E. (1996). "Intravenous and Buccal 2-Hydroxypropyl-β-Cyclodextrin Formulations of E2-CDS — Phase I Clinical Trials". Proceedings of the Eighth International Symposium on Cyclodextrins. pp. 507–510. doi:10.1007/978-94-011-5448-2_112. ISBN 978-0-7923-4029-4.
- Loftsson T, Gudmundsson JA, Arnadóttir RO, Fridriksdóttir H (May 2003). "Sublingual delivery of 17beta-estradiol from cyclodextrin containing tablets". Pharmazie. 58 (5): 358–9. PMID 12779059.
- Horský, Jan; Presl, Jiří (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In J. Horsky; J. Presl (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- Burnier AM, Martin PL, Yen SS, Brooks P (May 1981). "Sublingual absorption of micronized 17beta-estradiol". Am. J. Obstet. Gynecol. 140 (2): 146–50. doi:10.1016/0002-9378(81)90101-0. PMID 6786097.
- Fiet J, Hermano M, Witte J, Villette JM, Haimart M, Gourmel B, Tabuteau F, Rouffy J, Dreux C (September 1982). "Post-menopausal concentrations of plasma oestradiol, oestrone, FSH and LH and of total urinary oestradiol and oestrone after a single sublingual dose of oestradiol-17 beta". Acta Endocrinol. 101 (1): 93–7. doi:10.1530/acta.0.1010093. PMID 6812348.
- Jain J, Kwan D, Forcier M (April 2019). "Medroxyprogesterone Acetate in Gender-Affirming Therapy for Transwomen: Results from a Retrospective Study". The Journal of Clinical Endocrinology & Metabolism. doi:10.1210/jc.2018-02253/5479358. ISSN 0021-972X. PMID 31127826.
- Devissaguet JP, Brion N, Lhote O, Deloffre P (1999). "Pulsed estrogen therapy: pharmacokinetics of intranasal 17-beta-estradiol (S21400) in postmenopausal women and comparison with oral and transdermal formulations". Eur J Drug Metab Pharmacokinet. 24 (3): 265–71. doi:10.1007/BF03190030. PMID 10716066.
- Dooley, Mukta; Spencer, Caroline M.; Ormrod, Douglas (2001). "Estradiol-Intranasal". Drugs. 61 (15): 2243–2262. doi:10.2165/00003495-200161150-00012. ISSN 0012-6667. PMID 11772138.
- Lopes P, Rozenberg S, Graaf J, Fernandez-Villoria E, Marianowski L (2001). "Aerodiol versus the transdermal route: perspectives for patient preference". Maturitas. 38 Suppl 1: S31–9. doi:10.1016/S0378-5122(01)00202-X. PMID 11390122.
- Jeffrey K. Aronson (21 February 2009). Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. pp. 173–. ISBN 978-0-08-093292-7.
- Janice Ryden; Paul D. Blumenthal (2002). Practical Gynecology: A Guide for the Primary Care Physician. ACP Press. pp. 436–. ISBN 978-0-943126-94-4.
- Sahin FK, Koken G, Cosar E, Arioz DT, Degirmenci B, Albayrak R, Acar M (2008). "Effect of Aerodiol administration on ocular arteries in postmenopausal women". Gynecol. Endocrinol. 24 (4): 173–7. doi:10.1080/09513590701807431. PMID 18382901.
300 μg 17β-estradiol (Aerodiol®; Servier, Chambrayles-Tours, France) was administered via the nasal route by a gynecologist. This product is unavailable after March 31, 2007 because its manufacturing and marketing are being discontinued.
- "Aerodiol nasal spray (discontinued in the UK - December 2006)". Netdoctor. 19 January 2007.
- http://www.medicines.org.au/files/secaerod.pdf
- Comprehensive Toxicology. Elsevier Science. 1 December 2017. pp. 2–. ISBN 978-0-08-100612-2.
- James M. Rippe (15 March 2013). Lifestyle Medicine, Second Edition. CRC Press. pp. 279–. ISBN 978-1-4398-4542-4.
- Buster JE (June 2010). "Transdermal menopausal hormone therapy: delivery through skin changes the rules". Expert Opin Pharmacother. 11 (9): 1489–99. doi:10.1517/14656561003774098. PMID 20426703.
- Vinod P. Shah; Howard I. Maibach (29 June 2013). Topical Drug Bioavailability, Bioequivalence, and Penetration. Springer Science & Business Media. pp. 47–50. ISBN 978-1-4899-1262-6.
- Sitruk-Ware, Régine (1993). "Percutaneous and Transdermal Oestrogen Replacement Therapy". Annals of Medicine. 25 (1): 77–82. doi:10.3109/07853899309147862. ISSN 0785-3890. PMID 8435193.
- Sitruk-Ware R (January 1989). "Transdermal delivery of steroids". Contraception. 39 (1): 1–20. doi:10.1016/0010-7824(89)90012-7. PMID 2642780.
- Wiedersberg S, Guy RH (September 2014). "Transdermal drug delivery: 30+ years of war and still fighting!" (PDF). J Control Release. 190: 150–6. doi:10.1016/j.jconrel.2014.05.022. PMID 24852092.
- "EstroGel® 0.06% (Estradiol Gel) for Topical Use FDA Label" (PDF). Food and Drug Administration. 2017. Retrieved 22 July 2018.
- Schenkel, Lotte; Barlier, Danielle; Riera, Margrit; Barner, Andreas (1986). "Transdermal absorption of estradiol 101 from different body sites is comparable". Journal of Controlled Release. 4 (3): 195–201. doi:10.1016/0168-3659(86)90003-9. ISSN 0168-3659.
- Balfour, Julia A.; Heel, Rennie C. (1990). "Transdermal Estradiol". Drugs. 40 (4): 561–582. doi:10.2165/00003495-199040040-00006. ISSN 0012-6667. PMID 2083514.
- Kelly Smith; Daniel M. Riche; Nickole Henyan (15 April 2010). Clinical Drug Data, 11th Edition. McGraw Hill Professional. ISBN 978-0-07-162686-6.
- Feldmann RJ, Maibach HI (February 1967). "Regional variation in percutaneous penetration of 14C cortisol in man". J. Invest. Dermatol. 48 (2): 181–3. doi:10.1038/jid.1967.29. PMID 6020682.
- Iyer R, Mok SF, Savkovic S, Turner L, Fraser G, Desai R, Jayadev V, Conway AJ, Handelsman DJ (July 2017). "Pharmacokinetics of testosterone cream applied to scrotal skin". Andrology. 5 (4): 725–731. doi:10.1111/andr.12357. PMID 28334510.
- Kühnert B, Byrne M, Simoni M, Köpcke W, Gerss J, Lemmnitz G, Nieschlag E (August 2005). "Testosterone substitution with a new transdermal, hydroalcoholic gel applied to scrotal or non-scrotal skin: a multicentre trial". Eur. J. Endocrinol. 153 (2): 317–26. doi:10.1530/eje.1.01964. PMID 16061839.
- Berth-Jones, John (2016). "Principles of Topical Therapy". Rook's Textbook of Dermatology. pp. 1–51. doi:10.1002/9781118441213.rtd0018. ISBN 9781118441213.
- Benedetti, Margherita Strolin; Whomsley, Rhys; Poggesi, Italo; Cawello, Willi; Mathy, François-Xavier; Delporte, Marie-Laure; Papeleu, Peggy; Watelet, Jean-Baptiste (2009). "Drug metabolism and pharmacokinetics". Drug Metabolism Reviews. 41 (3): 344–390. doi:10.1080/10837450902891295. ISSN 0360-2532. PMC 3086155. PMID 19601718.
- Ronald C. Wester; Howard I. Maibach (2 January 2002). "Regional Variation in Percutaneous Absorption". In Robert L. Bronaugh; Howard I. Maibach (eds.). Topical Absorption of Dermatological Products. CRC Press. pp. 33–42. doi:10.3109/9780203904015-6. ISBN 978-0-203-90401-5.
- Re I, Asenjo G, Maximino G, Micheletti L (2005). "Tratamiento del Cáncer de Próstata Avanzado con Estrógenos Transdérmicos Escrotales (ETE)" [Transdermal Scrotal Estrogen Patches (TSEP) in the Treatment of Advanced Prostate Cancer]. Revista Argentina de Urología (in Spanish). 70 (4): 231. ISSN 0325-2531.
- Wisner KL, Sit DK, Moses-Kolko EL, Driscoll KE, Prairie BA, Stika CS, Eng HF, Dills JL, Luther JF, Wisniewski SR (August 2015). "Transdermal Estradiol Treatment for Postpartum Depression: A Pilot, Randomized Trial". J Clin Psychopharmacol. 35 (4): 389–95. doi:10.1097/JCP.0000000000000351. PMC 4485597. PMID 26061609.
Berlex-sponsored pharmacokinetic studies of the Climara® E2 transdermal system demonstrated an approximate 1:1 ratio of [E2 in mcg] delivered to [serum E2 pg/ml concentration] in menopausal women) [11]. Therefore, we anticipated that E2 doses of 50, 100, 150 and 200 mcg/day would produce 50, 100, 150, and 200 pg/ml serum concentrations.
- John J. Mulcahy (16 November 2007). Male Sexual Function: A Guide to Clinical Management. Springer Science & Business Media. pp. 309–. ISBN 978-1-59745-155-0.
- Lobo, Rogerio A. (2011). "Risk of venous thromboembolism by route of administration of estrogen". Menopause. 18 (5): 469–470. doi:10.1097/gme.0b013e318211745b. ISSN 1072-3714. PMID 21407136.
- Renoux, C.; Dell'Aniello, S.; Garbe, E.; Suissa, S. (2010). "Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study". BMJ. 340 (jun03 4): c2519. doi:10.1136/bmj.c2519. ISSN 0959-8138. PMID 20525678.
- Vinogradova, Yana; Coupland, Carol; Hippisley-Cox, Julia (2019). "Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases". BMJ. 364: k4810. doi:10.1136/bmj.k4810. ISSN 0959-8138. PMC 6326068. PMID 30626577.
- "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 26 July 2018.
- Langley RE, Godsland IF, Kynaston H, Clarke NW, Rosen SD, Morgan RC, Pollock P, Kockelbergh R, Lalani EN, Dearnaley D, Parmar M, Abel PD (August 2008). "Early hormonal data from a multicentre phase II trial using transdermal oestrogen patches as first-line hormonal therapy in patients with locally advanced or metastatic prostate cancer". BJU Int. 102 (4): 442–5. doi:10.1111/j.1464-410X.2008.07583.x. PMC 2564109. PMID 18422771.
- Deutsch MB, Bhakri V, Kubicek K (2015). "Effects of cross-sex hormone treatment on transgender women and men". Obstet Gynecol. 125 (3): 605–10. doi:10.1097/AOG.0000000000000692. PMC 4442681. PMID 25730222.
- Kumar C, McIvor RJ, Davies T, Brown N, Papadopoulos A, Wieck A, Checkley SA, Campbell IC, Marks MN (February 2003). "Estrogen administration does not reduce the rate of recurrence of affective psychosis after childbirth". J Clin Psychiatry. 64 (2): 112–8. doi:10.4088/JCP.v64n0202. PMID 12633118.
- Justice, Angela J.H.; de Wit, Harriet (2000). "Acute Effects of Estradiol Pretreatment on the Response to d-Amphetamine in Women". Neuroendocrinology. 71 (1): 51–59. doi:10.1159/000054520. ISSN 0028-3835. PMID 10644899.
- Lycette JL, Bland LB, Garzotto M, Beer TM (December 2006). "Parenteral estrogens for prostate cancer: can a new route of administration overcome old toxicities?". Clin Genitourin Cancer. 5 (3): 198–205. doi:10.3816/CGC.2006.n.037. PMID 17239273.
- "Prostate Adenocarcinoma TransCutaneous Hormones (PATCH)". ClinicalTrials.gov. U.S. National Library of Medicine. Retrieved 21 June 2019.
- Gilbert, Duncan C.; Duong, Trinh; Sydes, Matthew; Bara, Anna; Clarke, Noel; Abel, Paul; James, Nick; Langley, Ruth; Parmar, Max (2018). "Transdermal oestradiol as a method of androgen suppression for prostate cancer within the STAMPEDE trial platform" (PDF). BJU International. 121 (5): 680–683. doi:10.1111/bju.14153. ISSN 1464-4096. PMID 29388336.
- Singla, Nirmish; Ghandour, Rashed A.; Raj, Ganesh V. (2019). "Investigational luteinizing hormone releasing hormone (LHRH) agonists and other hormonal agents in early stage clinical trials for prostate cancer". Expert Opinion on Investigational Drugs. 28 (3): 249–259. doi:10.1080/13543784.2019.1570130. ISSN 1354-3784. PMID 30649971.
- "Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE)". ClinicalTrials.gov. U.S. National Library of Medicine. Retrieved 21 June 2019.
- Dahl, Marshall; Feldman, Jamie L.; Goldberg, Joshua M.; Jaberi, Afshin (2006). "Physical Aspects of Transgender Endocrine Therapy". International Journal of Transgenderism. 9 (3–4): 111–134. doi:10.1300/J485v09n03_06. ISSN 1553-2739.
- Elami-Suzin, Matan; Schenker, Joseph G. (2017). "Adolescent Pregnancy and Contraception". Frontiers in Gynecological Endocrinology. ISGE Series. pp. 199–227. doi:10.1007/978-3-319-41433-1_14. ISBN 978-3-319-41431-7. ISSN 2197-8735.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20323S23lbl.pdf
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020538s035lbl.pdf
- https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d28bec8f-762e-4f05-a20d-96a42970d6a7
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020375s034lbl.pdf
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020375s035lbl.pdf
- https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e7e6da3b-8485-1382-61c9-e9b369018b98
- "Climara Forte" (HTML). HPRA. Retrieved 22 December 2018.
- Ockrim J, Lalani EN, Abel P (October 2006). "Therapy Insight: parenteral estrogen treatment for prostate cancer--a new dawn for an old therapy". Nat Clin Pract Oncol. 3 (10): 552–63. doi:10.1038/ncponc0602. PMID 17019433.
- Norman G, Dean ME, Langley RE, Hodges ZC, Ritchie G, Parmar MK, Sydes MR, Abel P, Eastwood AJ (February 2008). "Parenteral oestrogen in the treatment of prostate cancer: a systematic review". Br. J. Cancer. 98 (4): 697–707. doi:10.1038/sj.bjc.6604230. PMC 2259178. PMID 18268497.
- Steg A, Benoit G (October 1979). "Percutaneous 17 beta-estradiol in treatment of cancer of prostate". Urology. 14 (4): 373–5. doi:10.1016/0090-4295(79)90083-9. ISSN 0090-4295. PMID 227153.
- Steg A, Benoit G, Limouzin-Lamotte A, Mahoudeau J, Caillens M, Raichvarg D (1979). "Cancer de la prostate: effets métaboliques des bêta-estradiol par voie percutanée" [Cancer of the prostate: metabolic effect of percutaneous beta-estradiol]. La Nouvelle Presse Medicale. 8 (46): 3801–3802. ISSN 0035-3655.
- Steg A, Benoit G, Limouzin-Lamotte A, Mahoudeau J, Caillens M, Raichvarg D (November 1980). "Cancer de la prostate: effets métaboliques du bêta-estradiol par voie percutanée" [Cancer of the prostate: metabolic effects of percutaneously administered beta-estradiol]. Rev Med Suisse Romande (in French). 100 (11): 895–7. ISSN 0035-3655. PMID 7466061.
- Steg A, Benoit G, Maisonneuve P, Tallet F, Nahoul K, Sulatna Y, Raichwarg D, Limousinlamotte MA (1983). "Étude Comparative du Diéthylstilboestrol et du 17 Bêta-oestradiol Per-cutané dans le Traitement du Cancer de la Prostate" [A comparative study of percutaneous 17 beta-estradiol and diethylstilbestrol in the treatment of prostatic cancer]. Ann Urol (Paris) (in French). 17: 197–202. ISSN 0003-4401.
- Steg A, Benoit G (1983). "Étude comparative de fortes doses d'oestradiol-17β administrées par voie per-cutanée et de l'orchidectomie bilatérale dans le traitement du cancer de la prostate" [Prostatic carcinoma. Bilateral orchiectomy versus percutaneous administration of large doses of 17β-estradiol. A comparative study.]. Annales d'Urologie. 17 (5): 286–288. ISSN 0003-4401.
- Benoit G (1985). "Que Penser du Traitement Hormonal du Cancer de la Prostate" [Thoughts on the Hormonal Treatment of Prostate Cancer]. Gazette Médicale. 92 (5): 33–39. ISSN 0760-758X.
- William H. Hindle (1998). "Structure-function relationships and metabolism of estrogens and progestogens". In Ian S. Fraser; R. P. S. Jansen; R. A. Lobo; M. I. Whitehead (eds.). Estrogens and Progestogens in Clinical Practice. Churchill Livingstone. pp. 187–194. ISBN 978-0-443-04706-0.
- Chang KJ, Lee TT, Linares-Cruz G, Fournier S, de Ligniéres B (April 1995). "Influences of percutaneous administration of estradiol and progesterone on human breast epithelial cell cycle in vivo". Fertil. Steril. 63 (4): 785–91. doi:10.1016/S0015-0282(16)57482-2. PMID 7890063.
- Spicer DV, Ursin G, Pike MC (May 1996). "Progesterone concentrations--physiologic or pharmacologic?". Fertil. Steril. 65 (5): 1077–8. doi:10.1016/s0015-0282(16)58295-8. PMID 8612843.
- Foidart JM, Colin C, Denoo X, Desreux J, Béliard A, Fournier S, de Lignières B (May 1998). "Estradiol and progesterone regulate the proliferation of human breast epithelial cells". Fertil. Steril. 69 (5): 963–9. doi:10.1016/s0015-0282(98)00042-9. PMID 9591509.
- Willibald Pschyrembel (1968). Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. pp. 598–. ISBN 978-3-11-150424-7.
- Shahrokh Tehraninejad E, Kabodmehri R, Hosein Rashidi B, Jafarabadi M, Keikha F, Masomi M, Hagholahi F (January 2018). "Trans dermal estrogen (oestrogel) for endometrial preparation in freeze embryo transfer cycle: An RCT". Int J Reprod Biomed (Yazd). 16 (1): 51–56. doi:10.29252/ijrm.16.1.51. PMC 5899770. PMID 29675488.
- Scott RT, Ross B, Anderson C, Archer DF (May 1991). "Pharmacokinetics of percutaneous estradiol: a crossover study using a gel and a transdermal system in comparison with oral micronized estradiol". Obstet Gynecol. 77 (5): 758–64. doi:10.1016/0020-7292(92)90761-7. PMID 2014092.
- Järvinen A, Granander M, Nykänen S, Laine T, Geurts P, Viitanen A (November 1997). "Steady-state pharmacokinetics of oestradiol gel in post-menopausal women: effects of application area and washing". Br J Obstet Gynaecol. 104 Suppl 16: 14–8. doi:10.1111/j.1471-0528.1997.tb11562. PMID 9389778.
- Manfred Kaufmann; Serban-Dan Costa; Anton Scharl (27 November 2013). Die Gynäkologie. Springer-Verlag. pp. 106–. ISBN 978-3-662-11496-4.
- Hall G, Blombäck M, Landgren BM, Bremme K (2002). "Effects of vaginally administered high estradiol doses on hormonal pharmacokinetics and hemostasis in postmenopausal women". Fertil. Steril. 78 (6): 1172–7. doi:10.1016/s0015-0282(02)04285-1. PMID 12477507.
- Schiff I, Tulchinsky D, Ryan KJ (October 1977). "Vaginal absorption of estrone and 17beta-estradiol". Fertil. Steril. 28 (10): 1063–6. doi:10.1016/S0015-0282(16)42855-4. PMID 908445.
- Vaughn TC, Hammond CB (March 1981). "Estrogen replacement therapy". Clin Obstet Gynecol. 24 (1): 253–83. doi:10.1097/00003081-198103000-00023. PMID 6783358.
- Edman, Clare D. (1983). "Estrogen Replacement Therapy". In J.J. Buchsbaum (ed.). The Menopause. pp. 77–84. doi:10.1007/978-1-4612-5525-3_6. ISBN 978-1-4612-5525-3. ISSN 0178-0328.
- Rigg LA, Milanes B, Villanueva B, Yen SS (December 1977). "Efficacy of intravaginal and intranasal administration of micronized estradiol-17beta". J. Clin. Endocrinol. Metab. 45 (6): 1261–4. doi:10.1210/jcem-45-6-1261. PMID 591620.
- M.R. Henzl (1978). "Natural and Synthetic Female Sex Hormones". In S.S.C. Yen; R.B. Jaffe (eds.). Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. W.B. Saunders Co. pp. 421–468. ISBN 978-0721696256.
- Lauritzen C (December 1986). "Die Behandlung der klimakterischen Beschwerden durch vaginale, rektale und transdermale Ostrogensubstitution" [Treatment of disorders of the climacteric by vaginal, rectal and transdermal estrogen substitution]. Gynakologe (in German). 19 (4): 248–53. ISSN 0017-5994. PMID 3817597.
- Lauritzen, C. (1987). "Neue Gesichtspunkte in der Behandlung postmenopausaler Beschwerden" [New aspects in the treatment of postmenopausal complaints]. Archives of Gynecology and Obstetrics (in German). 242 (1–4): 471–479. doi:10.1007/BF01783219. ISSN 0932-0067. PMID 3688962.
- Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- Göretzlehner G (1989). "Wirkungsstärke unterschiedlicher Estrogene in Abhängigkeit vom Applikationsmodus" [Efficacy of different estrogens as subject to mode of application]. Zentralbl Gynakol (in German). 111 (16): 1093–100. ISSN 0044-4197. PMID 2683509.
- Nizza M, Giardinelli M (August 1954). "Studio dell'attività degli estrogeni naturali e sintetici somministrati per via rettale mediante il controllo degli strisci vaginali" [Activity of natural and synthetic estrogens administered by the rectal route: study by vaginal smears]. Minerva Ginecol (in Italian). 6 (15): 548–51. ISSN 0026-4784. PMID 13203215.
- A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 548, 551. ISBN 978-3-642-96158-8.
- Moran DJ, McGarrigle HH, Lachelin GC (January 1994). "Maternal plasma progesterone levels fall after rectal administration of estriol". J. Clin. Endocrinol. Metab. 78 (1): 70–2. doi:10.1210/jcem.78.1.8288717. PMID 8288717.
- Anthony W. Norman; Gerald Litwack (23 October 1997). Hormones. Academic Press. pp. 398–. ISBN 978-0-08-053413-8.
- Asim Kurjak; Frank A. Chervenak (25 September 2006). Textbook of Perinatal Medicine, Second Edition. CRC Press. pp. 699–. ISBN 978-1-4398-1469-7.
- Michael F Greene; Robert K. Creasy; Robert Resnik; Jay D. Iams; Charles J. Lockwood; Thomas Moore (25 November 2008). Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice E-Book. Elsevier Health Sciences. pp. 115–. ISBN 978-1-4377-2135-5.
- Oriowo MA, Landgren BM, Stenström B, Diczfalusy E (April 1980). "A comparison of the pharmacokinetic properties of three estradiol esters". Contraception. 21 (4): 415–24. doi:10.1016/S0010-7824(80)80018-7. PMID 7389356.
- Nagrath Arun; Malhotra Narendra; Seth Shikha (15 December 2012). Progress in Obstetrics and Gynecology—3. Jaypee Brothers Medical Publishers Pvt. Ltd. pp. 416–418. ISBN 978-93-5090-575-3.
- Brown, J. B. (1957). "The relationship between urinary oestrogens and oestrogens produced in the body". Journal of Endocrinology. 16 (2): 202–212. doi:10.1677/joe.0.0160202. ISSN 0022-0795. PMID 13491750.
- Beer CT, Gallagher TF (May 1955). "Excretion of estrogen metabolites by humans. I. The fate of small doses of estrone and estradiol-17beta". J. Biol. Chem. 214 (1): 335–49. doi:10.0000/www.jbc.org/214/1/335 (inactive 2 December 2019). PMID 14367392.
- Lauritzen, Christian (1988). "Natürliche und Synthetische Sexualhormone – Biologische Grundlagen und Behandlungsprinzipien" [Natural and Synthetic Sexual Hormones – Biological Basis and Medical Treatment Principles]. In Hermann P. G. Schneider; Christian Lauritzen; Eberhard Nieschlag (eds.). Grundlagen und Klinik der Menschlichen Fortpflanzung [Foundations and Clinic of Human Reproduction] (in German). Walter de Gruyter. pp. 229–306. ISBN 978-3110109689. OCLC 35483492.
- Ulrich U, Pfeifer T, Lauritzen C (1994). "Rapid increase in lumbar spine bone density in osteopenic women by high-dose intramuscular estrogen-progestogen injections. A preliminary report". Horm. Metab. Res. 26 (9): 428–31. doi:10.1055/s-2007-1001723. PMID 7835827.
- https://web.archive.org/web/20190519104654/http://www.sukl.cz/download/pil/PI16221.pdf
- http://www.sukl.cz/download/pil/PI15789.pdf
- von Wattenwyl, H (1944), "Über eine neue Anwendungsart oestrogener Substanzen" [A new type of application of estrogenic substances], Schweiz. Med. Wochenschr. (in German), 74: 159–161
- Toppozada, Hussein K. (1950). "Oestrogenic Therapy with Prolonged Action". Obstetrical & Gynecological Survey. 5 (4): 531. doi:10.1097/00006254-195008000-00021. ISSN 0029-7828.
- Ferin J (January 1952). "Relative duration of action of natural and synthetic estrogens administered parenterally in women with estrogen deficiency". J. Clin. Endocrinol. Metab. 12 (1): 28–35. doi:10.1210/jcem-12-1-28. PMID 14907837.
- Field-Richards S (April 1955). "A preliminary series of cases of uterine hypoplasia treated by local injection of an oestrogenic emulsion". J Obstet Gynaecol Br Emp. 62 (2): 205–13. doi:10.1111/j.1471-0528.1955.tb14121.x. PMID 14368390.
Oestradiol monobenzoate or oestradiol diproprionate are slowly absorbed from oily solution after intramuscular injection and for this purpose are to be preferred to the unesterified form. As an even slower absorption of oestradiol monobenzoate can be obtained from an aqueous emulsion of this hormone (Lens, Overbeek and Polderman, 1949). Such a preparation for parenteral use was made available for this experiment by Messrs. Organon Laboratories Limited.
- Lens J, Overbeek GA, Polderman J (1949). "The effect of sex hormones in some organic solvents; emulsified in water". Acta Endocrinol. 2 (4): 396–404. doi:10.1530/acta.0.0020396. PMID 18140399.
- Ralph I. Dorfman (5 December 2016). Steroidal Activity in Experimental Animals and Man. Elsevier Science. pp. 40–. ISBN 978-1-4832-7299-3.
Ferin (1952) also studied duration of action in women with estrogen deficiency by recording the days of freedom from hot flushes. He rates estradiol-3-benzoate, estradiol-3-furoate, estradiol dipropionate, estradiol-17-caprylate, estradiol-3-benzoate-17-caprylate in oil, and finally estradiol-3-benzoate in emulsion or as microcrystals in that order of duration of action. After 10 mg. of each of the above preparations, a woman would typically remain free of symptoms for 10 days. This could, however, be as much as 50 days.
- Kaiser R (September 1961). "Die Östrogenausscheidung im Zyklus und nach Injektion von Östradiolestern. Ein Beitrag zur Therapie mit Depotöstrogenen" [Estrogen excretion during the cycle and after injection of estradiol esters. A contribution to therapy with depot estrogens]. Geburtshilfe Frauenheilkd (in German). 21: 868–78. ISSN 0016-5751. PMID 13750804.
- Kaiser, R (1962). "Über die Oestrogenausscheidung nach Injektion von Oestradiolestern" [Estrogen excretion after injection of estradiol esters]. Gewebs-und Neurohormone: Physiologie des Melanophorenhormons [Tissue and Neurohormones: Physiology of the Melanophore Hormone] (in German). Springer, Berlin, Heidelberg. pp. 227–232. doi:10.1007/978-3-642-86860-3_24. ISBN 978-3-540-02909-0.
- Shearman RP (June 1975). "The development of depot contraceptives". J. Steroid Biochem. 6 (6): 899–902. doi:10.1016/0022-4731(75)90323-4. PMID 1177432.
- Edkins, Robert Patrick (1959). "The Modification of the Duration of Drug Action". Journal of Pharmacy and Pharmacology. 11 (S1): 54T–66T. doi:10.1111/j.2042-7158.1959.tb10412.x. ISSN 0022-3573.
- Herrmann, U. (1958). "Abhängigkeit der durch Oestrogen- und Progesteron-Kristalle induzierten Abbruchblutung von der Korngröße". Gynecologic and Obstetric Investigation. 146 (4): 318–323. doi:10.1159/000306607. ISSN 1423-002X.
- d’Arcangues, Catherine; Snow, Rachel C. (1999). "Injectable Contraceptives". Fertility Control — Update and Trends. pp. 121–149. doi:10.1007/978-3-642-86696-8_6. ISBN 978-3-642-86698-2.
Chemists from 12 countries worldwide synthesized 230 ester oximes and esters of norethisterone and levonorgestrel and 72 esters of testosterone. After purification and formulation, these compounds were tested in rodents, and in sub-human primates for the most promising ones. From these biological studies, it emerged that levonorgestrel esters were usually longer acting than the norethisterone esters; aqueous suspensions were generally better than oily solutions; and the duration of action of the longest acting agents was highly dependent on the crystal size of their aqueous suspensions.
- Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–85. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
DMPA is a microcrystalline aqueous suspension of medroxyprogesterone acetate which is given by deep intramuscular injection. As a result of the very low solubility in aqueous solution, it provides very prolonged release from the depot site. [...] The exact formulation and the size of the microcrystals is most important for duration of action. The smaller particles are more rapidly dissolved than larger ones and, hence, MPA appears more rapidly in the circulation, with more rapid elimination from the body. This is also true for the once-a-month formulation, Cyclofem.
- Fraser, Ian S. (1989). "Systemic hormonal contraception by non-oral routes". In Marcus Filshie; John Guillebaud (eds.). Contraception: Science and Practice. Elsevier Science. pp. 109–125. ISBN 978-1-4831-6366-6.
The more traditional intramuscular injectable methods consist of steroid acetates or esters which have been formulated in oily solutions or microcrystalline suspensions. The steroid esters in oily solution appear to be distributed to storage sites in adipose tissue from which they are slowly released into the circulation. The active steroid moiety is then cleaved from the ester after which it is able to exert its biological effect. Microcrystalline suspensions remain as a depot at the site of injection and the active steroid or ester is slowly released from the surface of the crystals.
- William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 314–322. ISBN 978-0-9828280-1-4.
- "Juvenum E (estradiol) – Medicamentos PLM". Medicamentos PLM. Archived from the original on 17 September 2018. Retrieved 17 September 2018.
- "Juvenum E". Drugs.com.
- Mikkola A, Ruutu M, Aro J, Rannikko S, Salo J (1999). "The role of parenteral polyestradiol phosphate in the treatment of advanced prostatic cancer on the threshold of the new millennium". Ann Chir Gynaecol. 88 (1): 18–21. PMID 10230677.
- "All Medicines – Pharmanovia".
- Stege R, Gunnarsson PO, Johansson CJ, Olsson P, Pousette A, Carlström K (May 1996). "Pharmacokinetics and testosterone suppression of a single dose of polyestradiol phosphate (Estradurin) in prostatic cancer patients". Prostate. 28 (5): 307–10. doi:10.1002/(SICI)1097-0045(199605)28:5<307::AID-PROS6>3.0.CO;2-8. PMID 8610057.
- "Polyestradiol Phosphate". Drugs.com.
- Kohli, M.; Alikhan, M. A.; Spencer, H. J.; Carter, G. (2004). "Phase I trial of intramuscular estradiol valerate (I/M-E) in hormone refractory prostate cancer". Journal of Clinical Oncology. 22 (14_suppl): 4726. doi:10.1200/jco.2004.22.90140.4726. ISSN 0732-183X.
- Kuhli M, McClellan J (2001). "Parenteral estrogen therapy in advanced prostate cancer: retrospective analysis of intramuscular estradiol valerate in "hormone refractory" prostate disease". Proc Am Soc Clin Oncol. 20: 2407a. ISSN 0736-7589.
- Ryan CJ, Small EJ (December 2003). "Role of secondary hormonal therapy in the management of recurrent prostate cancer". Urology. 62 Suppl 1: 87–94. doi:10.1016/j.urology.2003.10.002. PMID 14747046.
The use of parenteral estradiol [valerate] in patients with progressive disease after secondary hormonal therapy resulted in PSA decreases in 5 of 5 patients without metastatic disease in a pilot study. In this same study, parenteral estradiol in combination with chemotherapy was also administered.49
- Kohli M (January 2006). "Phase II study of transdermal estradiol in androgen-independent prostate carcinoma". Cancer. 106 (1): 234–5, author reply 235. doi:10.1002/cncr.21528. PMID 16284988.
[...] we explored the effect of combination parenteral estrogen (intramuscular estradiol valerate) and chemotherapy on coagulation and plasminogen system activation in androgen independent stage in a Phase I trial.3 Our primary goal was to monitor coagulation and plasminogen system activation and was performed by using subclinical markers such as thrombin–antithrombin complex (TAT; reference range,1.0 – 4.1 μg/L) and quantitative D-dimer levels (QDD, range:0 – 250 ng/mL) as surrogate markers predictive for thrombohemorrhagic complications. Three escalating doses (10 mg, 20 mg, 40 mg) of intramuscular estradiol valerate were administered every 2 weeks in 12 patients. Before each estradiol valerate dose, these markers were measured, and patients with rising levels above baseline measurements were given once daily prophylaxis with 60 mg of low molecular weight heparin. We found that the majority of patients (10 of 12) had subclinical hemostatic activation as measured by rising plasma TAT and QDD levels after estradiol dosing, which returned to patient-specific baseline with daily prophylactic anticoagulation. No clinical thrombosis or hemorrhagic event was observed.
- Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- Schwartz MM, Soule SD (July 1955). "Estradiol 17-beta-cyclopentylpropionate, a longacting estrogen". Am. J. Obstet. Gynecol. 70 (1): 44–50. doi:10.1016/0002-9378(55)90286-6. PMID 14388061.
- Unger CA (December 2016). "Hormone therapy for transgender patients". Transl Androl Urol. 5 (6): 877–884. doi:10.21037/tau.2016.09.04. PMC 5182227. PMID 28078219.
- Al-Futaisi AM, Al-Zakwani IS, Almahrezi AM, Morris D (December 2006). "Subcutaneous administration of testosterone. A pilot study report". Saudi Med J. 27 (12): 1843–6. PMID 17143361.
- Olson J, Schrager SM, Clark LF, Dunlap SL, Belzer M (September 2014). "Subcutaneous Testosterone: An Effective Delivery Mechanism for Masculinizing Young Transgender Men". LGBT Health. 1 (3): 165–7. doi:10.1089/lgbt.2014.0018. PMID 26789709.
- Spratt DI, Stewart II, Savage C, Craig W, Spack NP, Chandler DW, Spratt LV, Eimicke T, Olshan JS (July 2017). "Subcutaneous Injection of Testosterone Is an Effective and Preferred Alternative to Intramuscular Injection: Demonstration in Female-to-Male Transgender Patients". J. Clin. Endocrinol. Metab. 102 (7): 2349–2355. doi:10.1210/jc.2017-00359. PMID 28379417.
- McFarland J, Craig W, Clarke NJ, Spratt DI (August 2017). "Serum Testosterone Concentrations Remain Stable Between Injections in Patients Receiving Subcutaneous Testosterone". J Endocr Soc. 1 (8): 1095–1103. doi:10.1210/js.2017-00148. PMC 5686655. PMID 29264562.
- Wilson DM, Kiang TK, Ensom MH (March 2018). "Pharmacokinetics, safety, and patient acceptability of subcutaneous versus intramuscular testosterone injection for gender-affirming therapy: A pilot study". Am J Health Syst Pharm. 75 (6): 351–358. doi:10.2146/ajhp170160. PMID 29367424.
- Singh GK, Turner L, Desai R, Jimenez M, Handelsman DJ (July 2014). "Pharmacokinetic-pharmacodynamic study of subcutaneous injection of depot nandrolone decanoate using dried blood spots sampling coupled with ultrapressure liquid chromatography tandem mass spectrometry assays". J. Clin. Endocrinol. Metab. 99 (7): 2592–8. doi:10.1210/jc.2014-1243. PMID 24684468.
- Chan VO, Colville J, Persaud T, Buckley O, Hamilton S, Torreggiani WC (June 2006). "Intramuscular injections into the buttocks: are they truly intramuscular?". Eur J Radiol. 58 (3): 480–4. doi:10.1016/j.ejrad.2006.01.008. PMID 16495027.
- Palma S, Strohfus P (November 2013). "Are IM injections IM in obese and overweight females? A study in injection technique". Appl Nurs Res. 26 (4): e1–4. doi:10.1016/j.apnr.2013.09.002. PMID 24156877.
- Kuhl, Herbert; Taubert, Hans-Dieter (1987). Das Klimakterium – Pathophysiologie, Klinik, Therapie [The Climacteric – Pathophysiology, Clinic, Therapy] (in German). Stuttgart, Germany: Thieme Verlag. p. 108. ISBN 978-3137008019.
- Kuhl H (September 1990). "Pharmacokinetics of oestrogens and progestogens". Maturitas. 12 (3): 171–97. doi:10.1016/0378-5122(90)90003-O. PMID 2170822.
- Sherif, Katherine (2013). "The Significance of Hormone Routes of Administration". Hormone Therapy. pp. 57–61. doi:10.1007/978-1-4614-6268-2_7. ISBN 978-1-4614-6267-5.
- Ian M. Symonds; Sabaratnam Arulkumaran (3 August 2013). Essential Obstetrics and Gynaecology E-Book. Elsevier Health Sciences. pp. 258–. ISBN 978-0-7020-5475-4.
- Leo Jr. Plouffe; Veronica A. Ravnikar; Leon Speroff; Nelson B. Watts (6 December 2012). Comprehensive Management of Menopause. Springer Science & Business Media. pp. 271–. ISBN 978-1-4612-4330-4.
- Christoffel Jos van Boxtel; Budiono Santoso; I. Ralph Edwards (2008). Drug Benefits and Risks: International Textbook of Clinical Pharmacology. IOS Press. pp. 769–. ISBN 978-1-58603-880-9.
- Martin Birkhauser; David Barlow; Morris Notelovitz; Margaret Rees (12 August 2005). Health Plan for the Adult Woman: Management Handbook. CRC Press. pp. 27–. ISBN 978-0-203-49009-9.
- Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. pp. 404–406. ISBN 978-3-88763-075-1. Retrieved 13 September 2012.
- Catriona Melville (22 September 2015). Sexual and Reproductive Health at a Glance. Wiley. pp. 108–. ISBN 978-1-119-23516-3.
- "Estradiol". Drugs.com.
- Pinkerton JV (December 2014). "What are the concerns about custom-compounded "bioidentical" hormone therapy?". Menopause. 21 (12): 1298–300. doi:10.1097/GME.0000000000000376. PMID 25387347.
- Pinkerton JV, Pickar JH (February 2016). "Update on medical and regulatory issues pertaining to compounded and FDA-approved drugs, including hormone therapy". Menopause. 23 (2): 215–23. doi:10.1097/GME.0000000000000523. PMC 4927324. PMID 26418479.
- Santoro N, Braunstein GD, Butts CL, Martin KA, McDermott M, Pinkerton JV (April 2016). "Compounded Bioidentical Hormones in Endocrinology Practice: An Endocrine Society Scientific Statement". J. Clin. Endocrinol. Metab. 101 (4): 1318–43. doi:10.1210/jc.2016-1271. PMID 27032319.
- Alex Khot; Andrew Polmear (18 November 2011). Practical General Practice E-Book: Guidelines for Effective Clinical Management. Elsevier Health Sciences. pp. 338–. ISBN 978-0-7020-4909-5.
- Kronenberg F (1990). "Hot flashes: epidemiology and physiology". Ann. N. Y. Acad. Sci. 592 (1): 52–86, discussion 123–33. Bibcode:1990NYASA.592...52K. doi:10.1111/j.1749-6632.1990.tb30316.x. PMID 2197954.
- Gearhart JP, Witherington R, Coleman CH (January 1981). "Subcutaneous estradiol pellet implantation in management of advanced prostatic carcinoma". Urology. 17 (1): 44–8. doi:10.1016/0090-4295(81)90010-8. ISSN 0090-4295. PMID 6161467.
- Okie MV, Carden ML, McGee HJ, Tracey EM (March 1951). "Estradiol pellet implantation in carcinoma of the prostate". N Y State J Med. 51 (5): 637–40. ISSN 0028-7628. PMID 14815766.
- Tracey EM (June 1952). "The use of estradiol pellets in the treatment of prostatic carcinoma; reference to variation in response to steroid therapy". J Int Coll Surg. 17 (6): 849–52. ISSN 0096-557X. PMID 14938629.
- Kearns WM (1942). "Treatment of cancer of the prostate with estrogens". Wisconsin Med. J. (41): 575. ISSN 0043-6542.
- Kearns, Walter. M. (1942). "Pellet Implantation of Hormones in Urology". Journal of Urology. 47 (5): 587–590. doi:10.1016/S0022-5347(17)70847-6. ISSN 0022-5347.
- Ufer, Joachim (1968). "Die therapeutische Anwendung der Gestagene beim Menschen" [Therapeutic Use of Progestagens in Humans]. Die Gestagene [Progestogens]. Springer-Verlag. pp. 1026–1124. doi:10.1007/978-3-642-99941-3_7. ISBN 978-3-642-99941-3.
Combination with intrauterine estrogen treatment: In order to achieve a growth effect on the uterus with relatively small doses of estrogens, individual authors [235,264] applied intrauterine or intramural crystal suspensions. In an effort to maintain a regular cycle during this regimen, Husslein and Gitsch [418] injected 30 times 10 mg progesterone parenterally 14 days after topical treatment with 3–10 mg estradiol crystal suspension. In this way, they believe they have achieved maximum uterine development with a minimum of hormones.
- Husslein H, Gitsch E (1951). "Uber die intrauterine Applikation von Ostrogenen in Kristallsuspensionsform" [Intrauterine application of estrogen crystals in suspension]. Zentralbl Gynakol (in German). 73 (14): 1219–24. PMID 14867680.
- Leyendecker G, Geppert G, Nocke W, Ufer J (May 1975). "Untersuchungen zur Pharmakokinetik von Östradiol-17β, Östradiol-benzoat, Östradiol-Valerianat un Östradiol-Undezylat bei der Frau: Der Verlauf der Konzentration von Östradiol-17β, Östron, LH und FSH im Serum" [Estradiol 17β, estrone, LH and FSH in serum after administration of estradiol 17β, estradiol benzoate, estradiol valeriate and estradiol undecylate in the female]. Geburtshilfe Frauenheilkd (in German). 35 (5): 370–4. ISSN 0016-5751. PMID 1150068.
- Goh HH, Chew PC, Karim SM, Ratnam SS (February 1980). "Control of gonadotrophin secretion by steroid hormones in castrated male transsexuals. I. Effects of oestradiol infusion on plasma levels of follicle-stimulating hormone and luteinizing hormone". Clin. Endocrinol. (Oxf). 12 (2): 165–75. doi:10.1111/j.1365-2265.1980.tb02131.x. PMID 6772356.
- Goh HH, Ratnam SS (October 1990). "Effect of estrogens on prolactin secretion in transsexual subjects". Arch Sex Behav. 19 (5): 507–16. doi:10.1007/BF02442351. PMID 2260915.
- Düsterberg B, Schmidt-Gollwitzer M, Hümpel M (1985). "Pharmacokinetics and biotransformation of estradiol valerate in ovariectomized women". Horm. Res. 21 (3): 145–54. doi:10.1159/000180039. PMID 2987096.
- Betty L. Gahart; Adrienne R. Nazareno; Meghan Ortega, RN (9 May 2018). Gahart's 2019 Intravenous Medications - E-Book: A Handbook for Nurses and Health Professionals. Elsevier Health Sciences. pp. 361–. ISBN 978-0-323-61273-9.
- Bhavnani BR, Stanczyk FZ (July 2014). "Pharmacology of conjugated equine estrogens: efficacy, safety and mechanism of action". J. Steroid Biochem. Mol. Biol. 142: 16–29. doi:10.1016/j.jsbmb.2013.10.011. PMID 24176763.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/010402s068lbl.pdf
- Bergenheim AT, Henriksson R (February 1998). "Pharmacokinetics and pharmacodynamics of estramustine phosphate". Clin Pharmacokinet. 34 (2): 163–72. doi:10.2165/00003088-199834020-00004. PMID 9515186.
- Scoccia B, Demir H, Elter K, Scommegna A (2009). "Successful medical management of post-hysteroscopic metroplasty bleeding with intravenous estrogen therapy: a report of two cases and review of the literature". J Minim Invasive Gynecol. 16 (5): 639–42. doi:10.1016/j.jmig.2009.05.012. PMID 19835811.
- Gunnarsson PO, Forshell GP (June 1984). "Clinical pharmacokinetics of estramustine phosphate". Urology. 23 (6 Suppl): 22–7. doi:10.1016/S0090-4295(84)80093-X. PMID 6375076.
- Loeser AA (February 1948). "The action of intravenously injected sex hormones and other substances on the blood flow in the human endometrium". J Obstet Gynaecol Br Emp. 55 (1): 17–22. doi:10.1111/j.1471-0528.1948.tb07044.x. PMID 18902558.
- Hertz R, Tullner WW (October 1949). "Intravenous administration of massive dosages of estrogen to the human subject; blood levels attained". Proc. Soc. Exp. Biol. Med. 72 (1): 187–91. doi:10.3181/00379727-72-17373. PMID 15391710.
- Tullner, W. W.; Young, J. P.; Hertz, R (1952). "Administration of Massive Dosage of Oestrogen to Breast and Prostatic Cancer Patients; Blood Levels Attained". Ciba Foundation Symposium - Steroid Hormones and Tumour Growth (Book I of Colloquia on Endocrinology, Vol. 1). Novartis Foundation Symposia. pp. 157–169. doi:10.1002/9780470718759.ch13. ISBN 9780470718759. ISSN 1935-4657.
- Gerhard Geppert (1975). Untersuchungen zur Pharmakokinetik von Östradiol-17β, Östradiol-Benzoat, Östradiol-Valerianat und Östradiol-Undezylat bei der Frau: der Verlauf der Konzentrationen von Östradiol-17β, Östron, LH und FSH im Serum [Studies on the pharmacokinetics of estradiol-17β, estradiol benzoate, estradiol valerate, and estradiol undecylate in women: the progression of serum estradiol-17β, estrone, LH, and FSH concentrations]. pp. 1–34. OCLC 632312599.
- Buchsbaum HJ, ed. (2012). The Menopause (Clinical Perspectives in Obstetrics and Gynecology). New York, NY: Springer Science & Business Media. p. 64. ISBN 9781461255253.
- Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric : the Journal of the International Menopause Society. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- "EC 2.4.1.17 – glucuronosyltransferase and Organism(s) Homo sapiens". BRENDA. Technische Universität Braunschweig. January 2018. Retrieved 10 August 2018.
Further reading
- Alfred S. Wolf; H.P.G. Schneider (1989). Östrogene in Diagnostik und Therapie [Estrogens in Diagnostics and Therapy]. Springer-Verlag. pp. 1–. ISBN 978-3-642-75101-1.
- Kuhl H (September 1990). "Pharmacokinetics of oestrogens and progestogens". Maturitas. 12 (3): 171–97. doi:10.1016/0378-5122(90)90003-O. PMID 2170822.
- Lobo RA, Cassidenti DL (January 1992). "Pharmacokinetics of oral 17 beta-estradiol". J Reprod Med. 37 (1): 77–84. PMID 1548642.
- O'Connell MB (September 1995). "Pharmacokinetic and pharmacologic variation between different estrogen products". J Clin Pharmacol. 35 (9S): 18S–24S. doi:10.1002/j.1552-4604.1995.tb04143.x. PMID 8530713.
- Michael Oettel; Ekkehard Schillinger (1999). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. ISBN 978-3-642-58616-3.
- Michael Oettel; Ekkehard Schillinger (1999). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. ISBN 978-3-642-60107-1.
- Kuhl H (2000). "Pharmacology of estradiol and estriol". Menopause Review. 5: 23–44.
- Ruggiero RJ, Likis FE (2002). "Estrogen: physiology, pharmacology, and formulations for replacement therapy". J Midwifery Womens Health. 47 (3): 130–8. doi:10.1016/S1526-9523(02)00233-7. PMID 12071379.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Barnes, Randall B.; Levrant, Seth G. (2007). "Pharmacology of Estrogens". Treatment of the Postmenopausal Woman. pp. 767–777. doi:10.1016/B978-012369443-0/50066-1. ISBN 9780123694430.
- Fruzzetti F, Trémollieres F, Bitzer J (2012). "An overview of the development of combined oral contraceptives containing estradiol: focus on estradiol valerate/dienogest". Gynecol. Endocrinol. 28 (5): 400–8. doi:10.3109/09513590.2012.662547. PMC 3399636. PMID 22468839.
- Stanczyk FZ, Archer DF, Bhavnani BR (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–27. doi:10.1016/j.contraception.2012.12.011. PMID 23375353.
_in_postmenopausal_women.png)