Allenolic acid
Allenolic acid, or allenoic acid, is a synthetic,[1] nonsteroidal estrogen discovered in 1947 or 1948 that, although studied clinically,[2] was never marketed.[3][4][5] It is an open-ring or seco-analogue of steroidal estrogens like estrone and equilenin.[6][7][8] The compound was named after Dr. Edgar Allen, one of the pioneers in estrogen research.[9][10] Although described as an estrogen, allenolic acid probably is totally inactive at the receptor, whereas a derivative, allenestrol (α,α-dimethyl-β-ethylallenolic acid), is reported to be a potent estrogen.[11] Another derivative of allenolic acid (specifically 6-methoxy-allenestrol), methallenestril (brand name Vallestril), is also a potent estrogen and, in contrast to allenolic acid and allenestrol, has been marketed.[12][13][14][15]
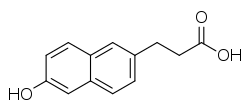 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H12O3 |
| Molar mass | 216.23258 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Rodolfo Paoletti; N. Pasetto; J.L. Ambrus (6 December 2012). The Menopause and Postmenopause: The Proceedings of an International Symposium held in Rome, June 1979. Springer Science & Business Media. pp. 110–. ISBN 978-94-011-7230-1.
- American Practitioner and Digest of Treatment. Lippincott. January 1951. p. 443.
- William J. Rea; Kalpana Patel (18 June 2010). Reversibility of Chronic Degenerative Disease and Hypersensitivity, Volume 1: Regulating Mechanisms of Chemical Sensitivity. CRC Press. pp. 464–. ISBN 978-1-4398-1345-4.
- Geynet, C.; Millet, C.; Truong, H.; Baulieu, E.E. (1972). "Estrogens and Antiestrogens". Gynecologic and Obstetric Investigation. 3 (1–4): 1–29. doi:10.1159/000301742. ISSN 1423-002X.
- Furuya, Hiroshi; Deguchi, Keiji; Shima, Motowo (1957). "Experimental and clinical studies on a new synthetic estrogen, an allenolic acid derivative, vallestril". American Journal of Obstetrics and Gynecology. 74 (3): 635–650. doi:10.1016/0002-9378(57)90519-7. ISSN 0002-9378.
- Indian Journal of Chemistry: Organic including medicinal. Council of Scientific & Industrial Research. 1980. p. 886.
- Paul Ghalioungui; Ahmed Ghareeb (1963). Endocrines, Vitamins, and Some Common Metabolic Disorders. Dar al-Maaref. p. 194.
- James D. Morrison (November 1983). Stereodifferentiating addition reactions. Academic Press. p. v. ISBN 978-0-12-507702-6.
- Willard Owen Thompson (1953). The Year Book of Endocrinology. Year Book Medical Publishers. p. 292.
- American Practitioner and Digest of Treatment. Lippincott. January 1956.
- Clark, Edward R.; Robson, R. D. (1959). "753. Oestrogenic carboxylic acids. Part II. Open-chain analogues of doisynolic acid". Journal of the Chemical Society (Resumed): 3714. doi:10.1039/jr9590003714. ISSN 0368-1769.
- Journal of the Japanese Obstetrical & Gynecological Society. 1958. p. 83.
- Erich Heftmann (1970). Steroid Biochemistry. Academic Press. p. 144.
- The Effects of the Sulfonylureas and Related Compounds in Experimental and Clinical Diabetes. The Academy. 1957. p. 681.
- Alan C. Sartorelli; David G. Johns (27 November 2013). Antineoplastic and Immunosuppressive Agents. Springer Science & Business Media. pp. 106–. ISBN 978-3-642-65806-8.