Paroxypropione
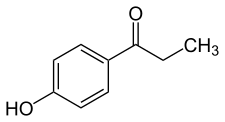
Paroxypropione, also known as paraoxypropiophenone, is a synthetic nonsteroidal estrogen which has been used medically as an antigonadotropin in Spain and Italy but appears to no longer be marketed.[1][2][3][4] It was first synthesized in 1902.[1] The antigonadotropic properties of the drug were discovered in 1951[3] and it entered clinical use shortly thereafter.[5]
 | |
| Clinical data | |
|---|---|
| Trade names | Frenantol, Frenormon, Hypophenon, Paroxon, Possipione, Profenone, others |
| Other names | Paraoxypropiophenone; H-365; NSC-2834; 4'-Hydroxypropiophenone; Ethyl p-hydroxyphenyl ketone; p-Propionylphenol; Paroxypropiophenone; Parahydroxypropiophenone; PHP |
| Drug class | Nonsteroidal estrogen; Antigonadotropin |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.676 |
| Chemical and physical data | |
| Formula | C9H10O2 |
| Molar mass | 150.175 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Pharmacology
Pharmacodynamics
Paroxypropione is closely related structurally to p-hydroxybenzoic acid and parabens such as methylparaben, and also bears a close resemblance to diethylstilbestrol (which, in fact, produces paroxypropione as an active metabolite)[6][7] and alkylphenols like nonylphenol, all of which are also estrogens.[8][9] The drug possesses relatively low affinity for the estrogen receptor[4] and must be given at high dosages to achieve significant estrogenic and antigonadotropic effects, for instance, 0.8 to 1.6 g/day.[10][11] It possesses 0.1% of the estrogenic activity and less than 0.5% of the antigonadotropic potency of estrone.[12]
Chemistry
Synthesis
The highest reported yield, approximately 96%, is from the between phenol and propionyl chloride.[13] The mechanism is likely to involve initial esterification to give phenyl propionate, which then undergoes a Fries rearrangement.
Derivatives
Paroxypropione is a precursor in the chemical synthesis of diethylstilbestrol and dienestrol.[14][15]
Society and culture
Names
Brand names Frenantol, Frenormon, Hypophenon, Paroxon, Possipione, Profenone, numerous others; former developmental code name NSC-2834), also known as paroxypropiophenone (P.O.P.) or 4'-hydroxypropiophenone.
References
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 662–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 796–. ISBN 978-3-88763-075-1.
- Paulsen, C. Alvin; Mortimore, Glenn E.; Heller, Carl G. (1951). "The Pituitary Action and Estrogenic Effect of Parahydroxypropiophenone". The Journal of Clinical Endocrinology & Metabolism. 11 (8): 892–894. doi:10.1210/jcem-11-8-892. ISSN 0021-972X. PMID 14861299.
- Mombelli, E. (2012). "Evaluation of the OECD (Q)SAR Application Toolbox for the profiling of estrogen receptor binding affinities". SAR and QSAR in Environmental Research. 23 (1–2): 37–57. doi:10.1080/1062936X.2011.623325. ISSN 1062-936X.
- Buu-Hoi, Ng. Ph.; Xuong, Ng. D.; Lavit, Denise (1953). "Fluorine-containing analogs of 4-hydroxypropiophenone". The Journal of Organic Chemistry. 18 (8): 910–915. doi:10.1021/jo50014a002. ISSN 0022-3263.
- P.L. Chambers; P. Günzel (12 March 2013). Mechanism of Toxic Action on Some Target Organs: Drugs and Other Substances. Springer Science & Business Media. pp. 276–. ISBN 978-3-642-67265-1.
- Gottschlich, Regina; Metzler, Manfred (1979). "High-pressure, reverse-phase partition chromatography separation of diethylstilbestrol metabolites and analogs". Analytical Biochemistry. 92 (1): 199–202. doi:10.1016/0003-2697(79)90645-6. ISSN 0003-2697.
- Richard E. Jones; Kristin H. Lopez (28 September 2013). Human Reproductive Biology. Academic Press. pp. 46–. ISBN 978-0-12-382185-0.
- Pugazhendhi D, Pope GS, Darbre PD (2005). "Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines". J Appl Toxicol. 25 (4): 301–9. doi:10.1002/jat.1066. PMID 16021681.
- De Vega Ruiz, T (1955). "Protein breakdown before and after operations. Influence of growth hormone and of inhibitors of the pituitary adrenal system". Cirug., Ginecol. Urol. 9: 289–326.
- Bussolati C, de Carneri I, Castellino S, Marinoni V, Sperzani GL (1967). "Treatment of Experimental and Clinical Schistosomiasis with Hormonal Inhibitors of Ovulation". Am. J. Trop. Med. Hyg. 16 (4): 497–9. doi:10.4269/ajtmh.1967.16.497. PMID 5006470.
- Scott, Charles C.; Kroc, Robert L.; Stasilli, Neil R. (1952). "Metabolic and Toxicity Studies on Para-Hydroxypropiophenone". Endocrinology. 50 (6): 607–611. doi:10.1210/endo-50-6-607. ISSN 0013-7227. PMID 12980070.
- Murashige, Ryo; Hayashi, Yuka; Ohmori, Syo; Torii, Ayuko; Aizu, Yoko; Muto, Yasuyuki; Murai, Yuta; Oda, Yuji; Hashimoto, Makoto (2011). "Comparisons of O-acylation and Friedel–Crafts acylation of phenols and acyl chlorides and Fries rearrangement of phenyl esters in trifluoromethanesulfonicacid: effective synthesis of optically active homotyrosines". Tetrahedron. 67 (3): 641–649. doi:10.1016/j.tet.2010.11.047. hdl:2115/44794.
- William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1286, 1290. ISBN 978-0-8155-1856-3.
- Harry H. Szmant (1989). Organic Building Blocks of the Chemical Industry. John Wiley & Sons. pp. 532–. ISBN 978-0-471-85545-3.
Further reading
- GUSTAVO RP (1958). "[Anti-gonadotropic action of possipione]". Quad Clin Ostet Ginecol (in Italian). 13 (7): 307–15. PMID 13579130.