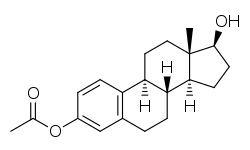
Estradiol acetate
Estradiol acetate (EA), sold under the brand names Femtrace, Femring, and Menoring, is an estrogen medication which is used in hormone therapy for the treatment of menopausal symptoms in women.[3][4][5][6] It is taken by mouth once daily or given as a vaginal ring once every three months.[2]
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌɛstrəˈdaɪoʊl ˈæsəteɪt/ ES-trə-DY-ohl ASS-ə-tayt[1] |
| Trade names | Femtrace, Femring, Menoring |
| Other names | EA; E2A; E3A; Estradiol 3-acetate |
| Routes of administration | By mouth, vaginal (ring)[2] |
| Drug class | Estrogen; Estrogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.110.039 |
| Chemical and physical data | |
| Formula | C20H26O3 |
| Molar mass | 314.419 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Side effects of estradiol acetate include breast tenderness, breast enlargement, nausea, headache, and fluid retention.[7][5][6] Estradiol acetate is a synthetic estrogen and hence is an agonist of the estrogen receptor, the biological target of estrogens like estradiol.[8][9] It is an estrogen ester and a prodrug of estradiol in the body.[9][8] Because of this, it is considered to be a natural and bioidentical form of estrogen.[9][10]
Estradiol acetate was introduced for medical use in 2001.[11] It is available in the United States and the United Kingdom.[11][3] The formulation for use by mouth has been discontinued in the United States.[12]
Medical uses
Estradiol acetate is used as a component of menopausal hormone therapy to treat and prevent menopausal symptoms such as hot flashes and osteoporosis in women.[13][14][15][16]
The Women's Health Initiative studies report increased health risks for menopausal women when using unopposed estrogens.[6] Estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.[6]
| Route/form | Estrogen | Low | Standard | High | |||
|---|---|---|---|---|---|---|---|
| Oral | Estradiol | 0.5–1 mg/day | 1–2 mg/day | 2–4 mg/day | |||
| Estradiol valerate | 0.5–1 mg/day | 1–2 mg/day | 2–4 mg/day | ||||
| Estradiol acetate | 0.45–0.9 mg/day | 0.9–1.8 mg/day | 1.8–3.6 mg/day | ||||
| Conjugated estrogens | 0.3–0.45 mg/day | 0.625 mg/day | 0.9–1.25 mg/day | ||||
| Esterified estrogens | 0.3–0.45 mg/day | 0.625 mg/day | 0.9–1.25 mg/day | ||||
| Estropipate | 0.75 mg/day | 1.5 mg/day | 3 mg/day | ||||
| Estriol | 1–2 mg/day | 2–4 mg/day | 4–8 mg/day | ||||
| Ethinylestradiola | – | 5–15 μg/day | – | ||||
| Nasal spray | Estradiol | 150 μg/day | 300 μg/day | 600 μg/day | |||
| Transdermal patch | Estradiol | 25 μg/dayb | 50 μg/dayb | 100 μg/dayb | |||
| Transdermal gel | Estradiol | 0.5 mg/day | 1–1.5 mg/day | 2–3 mg/day | |||
| Vaginal | Estradiol | 25 μg/day | – | – | |||
| Estriol | 30 μg/day | 0.5 mg 2x/week | 0.5 mg/day | ||||
| IM or SC injection | Estradiol valerate | – | – | 4 mg 1x/4 weeks | |||
| Estradiol cypionate | 1 mg 1x/3–4 weeks | 3 mg 1x/3–4 weeks | 5 mg 1x/3–4 weeks | ||||
| Estradiol benzoate | 0.5 mg 1x/week | 1 mg 1x/week | 1.5 mg 1x/week | ||||
| SC implant | Estradiol | 25 mg 1x/6 months | 50 mg 1x/6 months | 100 mg 1x/6 months | |||
| Footnotes: a = No longer used or recommended, due to health concerns. b = As a single patch applied once or twice per week (worn for 3–4 days or 7 days), depending on the formulation. Note: Dosages are not necessarily equivalent. Sources: See template. | |||||||
Available forms
Estradiol acetate comes in the form of 0.45, 0.9, and 1.8 mg oral tablets (Femtrace) and in the form of 12.4 or 24.8 mg vaginal rings that release 50 or 100 μg/day estradiol for 3 months (Femring, Menoring).[5][6][17] However, the Femtrace product was discontinued in the United States.[12]
| Route | Ingredient | Form | Dose | Major brand names |
|---|---|---|---|---|
| Oral | Estradiol | Tablet | 0.1, 0.2, 0.5, 1, 2, or 4 mg per tablet | Estrace, Ovocyclin |
| Estradiol acetatea | Tablet | 0.45, 0.9, or 1.8 mg per tablet | Femtrace | |
| Estradiol valerate | Tablet | 0.5, 1, 2, or 4 mg per tablet | Progynova | |
| Sublingual | Estradiola | Tablet | 0.125, 0.25, or 1 mg per tablet | Diogynets, Estradiol Membrettes |
| Intranasal | Estradiola | Nasal spray | 150 µg per spray (60 sprays per bottle) | Aerodiol |
| Transdermal | Estradiol | Patch | 14, 25, 37.5, 50, 60, 75, or 100 µg E2 per day for 3–4 or 7 days | Climara, Estraderm, Vivelle |
| Gel dispenser | 0.06% (0.87 or 1.25 g gel or 0.52 or 0.75 mg E2 per activation) | Elestrin, EstroGel | ||
| Gel packet | 0.1% (0.25, 0.5, or 1 g gel or 2.5, 5, or 10 mg E2 per packet) | DiviGel, Sandrena | ||
| Emulsion | 0.14% (1.74 g emulsion or 4.35 mg E2 per pouch; 50 µg/day E2) | Estrasorb | ||
| Spray | 1.53 mg per spray | Evamist | ||
| Vaginal | Estradiol | Tablet | 10 or 25 µg per tablet | Vagifem |
| Cream | 0.01% (0.1 mg E2 per 1 g cream) | Estrace | ||
| Suppositorya | 4 or 40 μg per suppository | Ovocyclin | ||
| Insert | 4 or 10 µg per insert (daily for 2 weeks then twice weekly) | Imvexxy | ||
| Ring | 2 mg per ring (7.5 µg/day E2 for 3 months) | Estring | ||
| Estradiol acetate | Ring | 12.4 or 24.8 mg per ring (50 or 100 µg/day E2 for 3 months) | Femring | |
| Injection (IM or SC) | Estradiol | Microspheres | 1 mg/mL | Juvenum E |
| Estradiol benzoate | Oil solution | 0.167, 0.2, 0.333, 1, 1.67, 2, 5, 10, 20, or 25 mg/mL | Progynon-B | |
| Aqueous suspensiona | 5 mg/mL | Agofollin Depot | ||
| Estradiol cypionate | Oil solution | 1, 3, or 5 mg/mL | Depo-Estradiol | |
| Aqueous suspension | 5 mg/0.5 mL (available only with a progestin) | Cyclofem, Lunelle | ||
| Estradiol dipropionatea | Oil solution | 0.1, 0.2, 0.5, 1, 2.5, or 5 mg/mL | Di-Ovocylin, Progynon-DP | |
| Estradiol enantate | Oil solution | 5 or 10 mg/mL (available only with a progestin) | Perlutal, Topasel | |
| Estradiol undecylatea | Oil solution | 100 mg/mL | Delestrec, Progynon Depot 100 | |
| Estradiol valerate | Oil solution | 5, 10, 20, or 40 mg/mL | Delestrogen, Progynon Depot | |
| Polyestradiol phosphatea | Aqueous solution | 40 or 80 mg per vial/ampoule | Estradurin | |
| Implant | Estradiola | Pellet | 20, 25, 50, or 100 mg per pellet (usually every 6 months) | Estradiol Implants, Meno-Implant |
| Abbreviations: E2 = Estradiol. Footnotes: a = Discontinued or mostly discontinued. Notes: (1): This table mostly does not include combination products, for instance estradiol formulated in combination with a progestogen or androgen. (2): This table does not include compounded estradiol products; only approved pharmaceutical preparations are included. (3): The availability of pharmaceutical estradiol products differs by country (see Estradiol (medication) § Availability). (4): Some of these formulations and doses have been marketed previously but may no longer be available. Sources: See template. | ||||
Contraindications
Contraindications of estrogens include coagulation problems, cardiovascular diseases, liver disease, and certain hormone-sensitive cancers such as breast cancer and endometrial cancer, among others.[18][19][20][21]
Side effects
The side effects of estradiol acetate are the same as those of estradiol. Examples of such side effects include breast tenderness and enlargement, nausea, bloating, edema, headache, and melasma.[7]
Overdose
Symptoms of estrogen overdosage may include nausea, vomiting, bloating, increased weight, water retention, breast tenderness, vaginal discharge, heavy legs, and leg cramps.[18] These side effects can be diminished by reducing the estrogen dosage.[18]
Interactions
Inhibitors and inducers of cytochrome P450 may influence the metabolism of estradiol and by extension circulating estradiol levels.[22]
Pharmacology
Pharmacodynamics
Estradiol acetate is an estradiol ester, or a prodrug of estradiol.[9][8] As such, it is an estrogen, or an agonist of the estrogen receptors.[8][9] Estradiol acetate is of about 15% higher molecular weight than estradiol due to the presence of its C3 acetate ester.[3] Because estradiol acetate is a prodrug of estradiol, it is considered to be a natural and bioidentical form of estrogen.[9][10]
Chemistry
Estradiol acetate is a synthetic estrane steroid and the C3 acetate ester of estradiol.[3] It is also known as estradiol 3-acetate or as estra-1,3,5(10)-triene-3,17β-diol 3-acetate.[3] Another common ester of estradiol in use for oral administration is estradiol valerate, which is a C17β ester of estradiol.[8][23]
| Estrogen | Structure | Ester(s) | Relative mol. weight | Relative E2 contentb | logPc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Estradiol | – | – | – | – | 1.00 | 1.00 | 4.0 | ||
| Estradiol acetate | C3 | Ethanoic acid | Straight-chain fatty acid | 2 | 1.15 | 0.87 | 2.8–3.9 | ||
| Estradiol benzoate | C3 | Benzenecarboxylic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.5–5.7 | ||
| Estradiol dipropionate | C3, C17β | Propanoic acid (×2) | Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.3 | ||
| Estradiol valerate | C17β | Pentanoic acid | Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.8–6.0 | ||
| Estradiol cypionate | C17β | Cyclopentylpropanoic acid | Aromatic fatty acid | – (~6) | 1.46 | 0.69 | 6.5–7.1 | ||
| Estradiol benzoate butyrate | C3, C17β | Benzoic acid, butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 5.9 | ||
| Estradiol enantate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | 7.0 | ||
| Estradiol dienantate | C3, C17β | Heptanoic acid (×2) | Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–9.1 | ||
| Estradiol undecylate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2 | ||
| Estradiol stearate | C17β | Octadecanoic acid | Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2 | ||
| Estradiol distearate | C3, C17β | Octadecanoic acid (×2) | Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | ||
| Estradiol sulfate | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | ||
| Estradiol glucuronide | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | ||
| Estramustine phosphated | C3, C17β | Normustine, phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | ||
| Polyestradiol phosphatee | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23f | 0.81f | 2.9g | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic fatty acids. b = Relative estradiol content by weight (i.e., relative estrogenic potency). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem and DrugBank. d = Also known as estradiol normustine phosphate. e = Polymer of estradiol phosphate (~13 repeat units). f = Relative molecular weight or estradiol content per repeat unit. g = logP of repeat unit (i.e., estradiol phosphate). Sources: See individual articles. | |||||||||
History
Estradiol acetate is relatively recent to the market, having been first approved in a vaginal ring formulation as Menoring in the United Kingdom in 2001,[13] followed by a vaginal ring formulation as Femring in the United States in 2002,[2] and finally as an oral preparation as Femtrace in the United States in 2004.[2][11]
Society and culture
References
- https://www.drugs.com/cdi/estradiol-acetate.html
- Sivanandy MS, Masimasi N, Thacker HL (May 2007). "Newer hormonal therapies: lower doses; oral, transdermal, and vaginal formulations". Cleveland Clinic Journal of Medicine. 74 (5): 369–75. doi:10.3949/ccjm.74.5.369. PMID 17506242.
- https://www.drugs.com/international/estradiol-acetate.html
- Buckler H, Al-Azzawi F (2003). "The effect of a novel vaginal ring delivering oestradiol acetate on climacteric symptoms in postmenopausal women". BJOG. 110 (8): 753–9. doi:10.1016/s1470-0328(03)02908-2. PMID 12892687.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021633s005lbl.pdf
- "FEMRING". DailyMed. U.S. National Library of Medicine.
- Amit K. Ghosh (23 September 2010). Mayo Clinic Internal Medicine Board Review. OUP USA. pp. 222–. ISBN 978-0-19-975569-1.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. p. 261. ISBN 978-3-642-60107-1.
Natural estrogens considered here include: [...] Esters of 17β-estradiol, such as estradiol valerate, estradiol benzoate and estradiol cypionate. Esterification aims at either better absorption after oral administration or a sustained release from the depot after intramuscular administration. During absorption, the esters are cleaved by endogenous esterases and the pharmacologically active 17β-estradiol is released; therefore, the esters are considered as natural estrogens.
- Cirigliano M (June 2007). "Bioidentical hormone therapy: a review of the evidence". J Womens Health (Larchmt). 16 (5): 600–31. doi:10.1089/jwh.2006.0311. PMID 17627398.
- Ballagh SA (2004). "Vaginal rings for menopausal symptom relief". Drugs Aging. 21 (12): 757–66. doi:10.2165/00002512-200421120-00001. PMID 15382956.
- https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021633
- Speroff L (October 2003). "Efficacy and tolerability of a novel estradiol vaginal ring for relief of menopausal symptoms". Obstetrics and Gynecology. 102 (4): 823–34. doi:10.1016/s0029-7844(03)00764-6. PMID 14551014.
- Al-Azzawi F, Lees B, Thompson J, Stevenson JC (2005). "Bone mineral density in postmenopausal women treated with a vaginal ring delivering systemic doses of estradiol acetate". Menopause. 12 (3): 331–9. doi:10.1097/01.gme.0000163870.03388.4d. PMID 15879923.
- Utian WH, Speroff L, Ellman H, Dart C (2005). "Comparative controlled trial of a novel oral estrogen therapy, estradiol acetate, for relief of menopause symptoms". Menopause. 12 (6): 708–15. doi:10.1097/01.gme.0000184220.63459.a8. PMID 16278614.
- Speroff L, Haney AF, Gilbert RD, Ellman H (2006). "Efficacy of a new, oral estradiol acetate formulation for relief of menopause symptoms". Menopause. 13 (3): 442–50. doi:10.1097/01.gme.0000182802.06762.b2. PMID 16735941.
- Deitra Leonard Lowdermilk; Shannon E. Perry; Mary Catherine Cashion; Kathryn Rhodes Alden (18 December 2014). Maternity and Women's Health Care - E-Book. Elsevier Health Sciences. pp. 137–. ISBN 978-0-323-39019-4.
- Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- Christian Lauritzen; John W. W. Studd (22 June 2005). Current Management of the Menopause. CRC Press. pp. 95–98, 488. ISBN 978-0-203-48612-2.
- Laurtizen, Christian (2001). "Hormone Substitution Before, During and After Menopause" (PDF). In Fisch, Franz H. (ed.). Menopause – Andropause: Hormone Replacement Therapy Through the Ages. Krause & Pachernegg: Gablitz. pp. 67–88. ISBN 978-3-901299-34-6.
- Midwinter, Audrey (1976). "Contraindications to estrogen therapy and management of the menopausal syndrome in these cases". In Campbell, Stuart (ed.). The Management of the Menopause & Post-Menopausal Years: The Proceedings of the International Symposium held in London 24–26 November 1975 Arranged by the Institute of Obstetrics and Gynaecology, The University of London. MTP Press Limited. pp. 377–382. doi:10.1007/978-94-011-6165-7_33. ISBN 978-94-011-6167-1.
- Cheng ZN, Shu Y, Liu ZQ, Wang LS, Ou-Yang DS, Zhou HH (February 2001). "Role of cytochrome P450 in estradiol metabolism in vitro". Acta Pharmacol. Sin. 22 (2): 148–54. PMID 11741520.
- Düsterberg B, Nishino Y (December 1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. doi:10.1016/0378-5122(82)90064-0. PMID 7169965.
- U.S. Food and Drug Administration (2009). Menopause - Medicines to Help You. GPO FCIC. pp. 3–. ISBN 978-1-61221-026-1.
- Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 757–. ISBN 978-1-4511-4847-3.