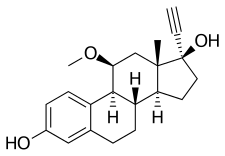
Moxestrol
Moxestrol, sold under the brand name Surestryl, is an estrogen medication which has been used in Europe for the treatment of menopausal symptoms and menstrual disorders.[3][4][2][5][6] It is taken by mouth.[6] In addition to its use as a medication, moxestrol has been used in scientific research as a radioligand of the estrogen receptor.[7]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Surestryl |
| Other names | R-2858, RU-2858, NSC-118191; 11β-Methoxy-17α-ethynylestradiol; 11β-MeO-EE 11β-Methoxy-17α-ethynylestra-1,3,5(10)-triene-3,17β-diol |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Estrogen; Estrogen ether |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 33%[1] |
| Protein binding | Minimal[1] |
| Metabolism | Liver[2] |
| Elimination half-life | 8.2 hours[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H26O3 |
| Molar mass | 326.429 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Medical uses
Moxestrol is or has been used in the treatment of menopausal symptoms and menstrual disorders.[2][6] It has been used at dosages of 50 to 150 µg per week for long-term therapy to 25 to 250 µg per day for short-term therapy.[6]
Pharmacology
Pharmacodynamics
Moxestrol is an estrogen, or an agonist of the estrogen receptors.[2][5] It is the 11β-methoxy derivative of ethinylestradiol and is one of the most potent estrogens known, being some 10 to 100 times more potent than estradiol and about 5-fold more potent than ethinylestradiol.[2][5] The very high potency of moxestrol has been attributed to its high affinity for the estrogen receptor (ER), its negligible plasma binding to sex hormone binding globulin and low binding to serum albumin,[1] and its lower relative rate of metabolism.[2][5] In contrast to estradiol, which has roughly the same affinity for both ERs (Ki = 0.12 nM and 0.15 nM, respectively), moxestrol possesses several-fold selectivity for the ERα (Ki = 0.50 nM) over ERβ (Ki = 2.6 nM).[8]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Estradiol | 2.6 | 7.9 | 100 | 0.6 | 0.13 | 8.7 | <0.1 |
| Ethinylestradiol | 15–25 | 1–3 | 112 | 1–3 | <1 | ? | ? |
| Moxestrol (11β-MeO-EE) | 0.8 | <0.1 | 12 | 3.2 | <0.1 | <0.2 | <0.1 |
| RU-16117 (11α-MeO-EE) | 1–3 | <1 | 13 | <1 | <1 | ? | ? |
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. Sources: [9][10][11][12] | |||||||
Pharmacokinetics
The bioavailability of moxestrol is 33%.[1] Its plasma protein binding is minimal.[1] The medication is metabolized in the liver.[2] Its biological half-life is 8.2 hours.[1]
Chemistry
Moxestrol, also known as 11β-methoxy-17α-ethynylestradiol (11β-MeO-EE) or as 11β-methoxy-17α-ethynylestra-1,3,5(10)-triene-3,17β-diol, is a synthetic estrane steroid and a derivative of estradiol.[3] It is specifically a derivative of ethinylestradiol (17α-ethynylestradiol) with a methoxy group at the C11β position.[3] The compound is the C11β isomer or C11 epimer of RU-16117 (11α-methoxy-17α-ethynylestradiol.[13]
Society and culture
Generic names
Moxestrol is the generic name of the drug and its INN.[3][4] It is also known by its developmental code name R-2858 or RU-2858.[3][4]
Availability
Moxestrol is or has been marketed in Europe.[2]
References
- Salmon, J.; Coussediere, D.; Cousty, C.; Raynaud, J.P. (1983). "Pharmacokinetics and metabolism of moxestrol in animals—rat, dog and monkey". Journal of Steroid Biochemistry. 19 (2): 1223–1234. doi:10.1016/0022-4731(83)90421-1. ISSN 0022-4731.
- Jonathan J. Li; Satyabrata Nandi; Sara A. Li (6 December 2012). Hormonal Carcinogenesis: Proceedings of the First International Symposium. Springer Science & Business Media. pp. 184–. ISBN 978-1-4613-9208-8.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 841–. ISBN 978-1-4757-2085-3.
- Dr. Ian Morton; I.K. Morton; Judith M. Hall (31 October 1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 186–. ISBN 978-0-7514-0499-9.
- Adrain D. Nunn (19 June 1992). Radiopharmaceuticals: Chemistry and Pharmacology. CRC Press. pp. 342–. ISBN 978-0-8247-8624-3.
- William Martindale; Royal Pharmaceutical Society of Great Britain. Dept. of Pharmaceutical Sciences (1993). The Extra Pharmacopoeia. Pharmaceutical Press. p. 1188. ISBN 978-0-85369-300-0.
Moxestrol is a synthetic oestrogen with actions and uses similar to thosre described for the oestrogens in general. Moxestrol is reponed to have a prolonged duration of action. It has been given by mouth in the treatment of menopausal, postmenopausal, and menstrual symptoms. Dose have ranged from 50 to 100 µg weekly for long-term therapy to 25 to 250 µg daily for short-term use.
- Raynaud JP, Martin PM, Bouton MM, Ojasoo T (September 1978). "11beta-Methoxy-17-ethynyl-1,3,5(10)-estratriene-3,17beta-diol (moxestrol), a tag for estrogen receptor binding sites in human tissues". Cancer Res. 38 (9): 3044–50. PMID 679210.
- Lund TD, Hinds LR, Handa RJ (2006). "The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus". J. Neurosci. 26 (5): 1448–56. doi:10.1523/JNEUROSCI.3777-05.2006. PMID 16452668.
- Raynaud, J.P.; Ojasoo, T.; Bouton, M.M.; Philibert, D. (1979). "Receptor Binding as a Tool in the Development of New Bioactive Steroids". Drug Design. pp. 169–214. doi:10.1016/B978-0-12-060308-4.50010-X. ISBN 9781483216102.
- Ojasoo T, Raynaud JP (November 1978). "Unique steroid congeners for receptor studies". Cancer Res. 38 (11 Pt 2): 4186–98. PMID 359134.
- Ojasoo T, Delettré J, Mornon JP, Turpin-VanDycke C, Raynaud JP (1987). "Towards the mapping of the progesterone and androgen receptors". J. Steroid Biochem. 27 (1–3): 255–69. doi:10.1016/0022-4731(87)90317-7. PMID 3695484.
- Raynaud JP, Bouton MM, Moguilewsky M, Ojasoo T, Philibert D, Beck G, Labrie F, Mornon JP (January 1980). "Steroid hormone receptors and pharmacology". J. Steroid Biochem. 12: 143–57. doi:10.1016/0022-4731(80)90264-2. PMID 7421203.
- Alvin M. Kaye; Myra Kaye (22 October 2013). Development of Responsiveness to Steroid Hormones: Advances in the Biosciences. Elsevier Science. pp. 61–. ISBN 978-1-4831-5308-7.