Estetrol (medication)
Estetrol (E4), tentative brand names Donesta (alone) and Estelle/PeriNesta (with drospirenone), is an estrogen medication and naturally occurring steroid hormone which is under development for use as a birth control pill in combination with a progestin, in menopausal hormone therapy to treat symptoms such as vaginal atrophy, hot flashes, and bone loss, and for the treatment of breast cancer, prostate cancer, osteoarthritis, and migraine.[3][4][1][2] It is taken by mouth.[3][4]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Donesta (alone), Estelle/PeriNesta (with drospirenone)[1][2] |
| Other names | Oestetrol; E4; 15α-Hydroxyestriol; Estra-1,3,5(10)-triene-3,15α,16α,17β-tetrol; Donesta; Estelle |
| Routes of administration | By mouth[3][4] |
| Drug class | Estrogen |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | High[5] |
| Protein binding | Moderately to albumin, not to SHBG[5][6] |
| Metabolism | Minimal, conjugation (glucuronidation, sulfation)[3][7] |
| Metabolites | Estetrol glucuronide[7][3] Estetrol sulfate[7] |
| Elimination half-life | Mean: 28 hours[5][7] Range: 18–60 hours[5] |
| Excretion | Urine: 79.7% (as conjugates)[3][7] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H24O4 |
| Molar mass | 304.386 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Solubility in water | 1.38 mg/mL (20 °C) |
SMILES
| |
InChI
| |
| (verify) | |
Estetrol is a naturally occurring and bioidentical estrogen, or an agonist of the estrogen receptor, the biological target of estrogens like endogenous estradiol.[3][4] Due to its estrogenic activity, estetrol has antigonadotropic effects and can inhibit fertility and suppress sex hormone production and levels in both women and men.[3][5][8] Estetrol differs in various ways both from other natural estrogens like estradiol and from synthetic estrogens like ethinylestradiol, with implications for tolerability and safety.[3][5] For instance, it appears to have minimal estrogenic effects in the breasts and liver.[3][5][9][7] Due to its unique tissue-selective effects, estetrol has been described as a natural selective estrogen receptor modulator (SERM).[3][10]
Estetrol was first discovered in 1965, and basic research continued up until 1984.[3][11] It started to be studied again as well as investigated for potential medical use in 2001, and by 2008, was of major interest for possible medical use.[3][4] As of 2018, estetrol is in mid- to late-stage clinical development for a variety of indications.[1][2] Mithra Pharmaceuticals anticipates the launch of Donesta and PeriNesta by the end of 2023.[1][2]
Medical uses
Estetrol is under development for use alone for a variety of indications with the tentative brand name Donesta.[1] Applications include hormonal contraception and menopausal hormone therapy among others.[3][4] As of June 2018, it is in phase II clinical trials for pregnancy prevention and the treatment of atrophic vaginitis, vasomotor symptoms, osteoarthritis, hormonal contraception, breast cancer, and prostate cancer, while it is in phase I clinical studies for the treatment of migraine.[1] It was also under development for the treatment of cardiovascular disorders, multiple sclerosis, and Sjogren's syndrome, but development for these indications was discontinued.[1]
In addition to a single-drug formulation, estetrol is under development in combination with the progestin drospirenone for use as a birth control pill to prevent pregnancy with the tentative brand name Estelle.[2] Drospirenone is also a potent antimineralocorticoid and antiandrogen in addition to progestogen, and in relation to this, is said to have a progesterone-like medication profile.[12][13][2] As of April 2018, estetrol/drospirenone are in phase III clinical trials for pregnancy prevention.[2]
Estetrol has been studied in humans at oral doses of 0.1 to 100 mg.[3][5][14] Dosages of between 2 and 40 mg/day estetrol have been studied in postmenopausal women and found to be effective in the alleviation of menopausal symptoms.[14]
| Route/form | Estrogen | Low | Standard | High | |||
|---|---|---|---|---|---|---|---|
| Oral | Estradiol | 0.5–1 mg/day | 1–2 mg/day | 2–4 mg/day | |||
| Estradiol valerate | 0.5–1 mg/day | 1–2 mg/day | 2–4 mg/day | ||||
| Estradiol acetate | 0.45–0.9 mg/day | 0.9–1.8 mg/day | 1.8–3.6 mg/day | ||||
| Conjugated estrogens | 0.3–0.45 mg/day | 0.625 mg/day | 0.9–1.25 mg/day | ||||
| Esterified estrogens | 0.3–0.45 mg/day | 0.625 mg/day | 0.9–1.25 mg/day | ||||
| Estropipate | 0.75 mg/day | 1.5 mg/day | 3 mg/day | ||||
| Estriol | 1–2 mg/day | 2–4 mg/day | 4–8 mg/day | ||||
| Ethinylestradiola | – | 5–15 μg/day | – | ||||
| Nasal spray | Estradiol | 150 μg/day | 300 μg/day | 600 μg/day | |||
| Transdermal patch | Estradiol | 25 μg/dayb | 50 μg/dayb | 100 μg/dayb | |||
| Transdermal gel | Estradiol | 0.5 mg/day | 1–1.5 mg/day | 2–3 mg/day | |||
| Vaginal | Estradiol | 25 μg/day | – | – | |||
| Estriol | 30 μg/day | 0.5 mg 2x/week | 0.5 mg/day | ||||
| IM or SC injection | Estradiol valerate | – | – | 4 mg 1x/4 weeks | |||
| Estradiol cypionate | 1 mg 1x/3–4 weeks | 3 mg 1x/3–4 weeks | 5 mg 1x/3–4 weeks | ||||
| Estradiol benzoate | 0.5 mg 1x/week | 1 mg 1x/week | 1.5 mg 1x/week | ||||
| SC implant | Estradiol | 25 mg 1x/6 months | 50 mg 1x/6 months | 100 mg 1x/6 months | |||
| Footnotes: a = No longer used or recommended, due to health concerns. b = As a single patch applied once or twice per week (worn for 3–4 days or 7 days), depending on the formulation. Note: Dosages are not necessarily equivalent. Sources: See template. | |||||||
Contraindications
General contraindications of estrogens include breast cancer, endometrial cancer, and others.[15]
Side effects
Minimal side effects have been observed with estetrol in women.[5][14] In men, decreased libido (in 40%) and nipple tenderness (in 35%) have been observed with high-dose (20–40 mg/day) estetrol for four weeks.[8] The medication poses a risk of endometrial hyperplasia and endometrial cancer in women similarly to other estrogens.[3][14] As such, it is necessary to combine estetrol with a progestogen in women with intact uteruses to prevent such risks.[10][14]
Overdose
High single doses of estetrol of 100 mg have been studied in women and were found to be well-tolerated.[5] Estetrol has been said to be only 10 to 20 times less potent orally than the highly potent estrogen ethinylestradiol.[5] During pregnancy, estetrol levels increase to high concentrations of about 723 pg/mL on average in the mother and about 9,034 pg/mL on average in the fetus (measured via umbilical cord blood) by term.[16] Estetrol levels are 10 to 20 times higher in the fetal circulation than in the maternal circulation (which is a consequence of the fact that estetrol is produced exclusively in the fetal liver).[5][16] The production of high amounts of estetrol during pregnancy suggests that it may be a reasonably safe compound at such concentrations.[17]
Interactions
Estetrol shows minimal to no inhibition or induction of cytochrome P450 enzymes.[3][18] In addition, estetrol undergoes minimal to no metabolism, aside from conjugation.[3][18] As such, estetrol is expected to harbor a low risk for drug interactions.[3][18]
Pharmacology
Pharmacodynamics
Estetrol is an agonist of the estrogen receptors (ERs), and hence is an estrogen.[3][4] It has moderate affinity for the ERα and ERβ, with Ki values of 4.9 nM and 19 nM, respectively.[3][18] As such, estetrol has 4- to 5-fold preference for the ERα over the ERβ.[3][18] For comparison, the potent nonsteroidal estrogen diethylstilbestrol showed higher affinities for the ERs, with Ki values of 0.286 nM for the ERα and 0.199 nM for the ERβ.[18] Similarly, estetrol has low affinity for the ERs relative to estradiol, and both estetrol and the related estrogen estriol require substantially higher concentrations than estradiol to produce similar effects to estradiol.[3] The affinity of estetrol for the ERs is about 0.3% (rat) to 6.25% (human) of that of estradiol, and its in vivo potency in animals is about 2 to 3% of that of estradiol.[3] In women, estetrol has been found to be approximately 10 to 20 times less potent orally than ethinylestradiol, the most potent oral estrogen available.[3] The high oral potency of estetrol in women in spite of relatively low affinity for the ERs is related to its high metabolic stability and favorable pharmacokinetics.[3]
Estetrol shows high selectivity for the ERs.[3][18] It showed only 11 to 15% occupation of the androgen, progesterone, and glucocorticoid receptors at a very high concentration of 10 μM.[3][18] In addition, estetrol showed no affinity (>10 μM) for a set of 124 receptors and enzymes, with the sole exception of very weak affinity for the α1B-adrenergic receptor (23% inhibition of prazosin binding at a concentration of 10 μM).[3][18] Due to its high selectivity for the ERs, estetrol is anticipated to have a low risk of undesirable off-target activity and associated side effects.[3][18] Furthermore, estetrol showed no inhibition of the major cytochrome P450 enzymes CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 at a very high concentration of 10 μM, unlike estradiol and ethinylestradiol.[3][18] Conversely, estetrol moderately stimulated CYP3A4 (by 23%), while estradiol strongly stimulated CYP3A4 (by 83%) and ethinylestradiol moderately inhibited the enzyme (by 45%).[3][18] These findings suggest that estetrol has a low potential for drug interactions, including a lower potential than estradiol and particularly ethinylestradiol.[3][18]
| RBA | RTC | |||
|---|---|---|---|---|
| Estrogen | ERα (%) | ERβ (%) | ERα (%) | ERβ (%) |
| Estradiol | 100 | 100 | 100 | 100 |
| Estrone | 4.0 | 3.5 | 2.6 | 4.3 |
| Estriol | 11.3 | 17.6 | 10.6 | 16.6 |
| Ethinylestradiol | 233 | 37.8 | 213 | 27.2 |
| Notes: At the human estrogen receptors. Sources: See template. | ||||
| Compound | PR | AR | ER | GR | MR | SHBG | CBG | ||
|---|---|---|---|---|---|---|---|---|---|
| Estradiol | 2.6 | 7.9 | 100 | 0.6 | 0.13 | 8.7–12 | <0.1 | ||
| Estrone | <1 | <1 | 35 | <1 | <1 | 2.7 | <0.1 | ||
| Estriol | <1 | <1 | 15 | <1 | <1 | <0.1 | <0.1 | ||
| Ethinylestradiol | 15–25 | 1–3 | 112 | 1–3 | <1 | 0.18 | <0.1 | ||
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. Sources: See template. | |||||||||
Differences from other estrogens
Estetrol has potent estrogenic effects in bone, vagina, uterus (both myometrium and endometrium), arteries, and certain areas of the brain like the pituitary gland and hypothalamus (in terms of hot flash relief, antigonadotropic effects, and ovulation inhibition).[3][19] It has comparable efficacy to ethinylestradiol on bone turnover and hot flashes and to estradiol valerate on vaginal atrophy.[3][7][14] In addition, estetrol has stimulatory effects on the endometrium and poses a risk of endometrial hyperplasia and endometrial cancer similarly to other estrogens.[3][14] Conversely, the effects of estetrol in certain other tissues such as breast/mammary gland, liver, vascular tissue, and various brain areas differ, with weakly estrogenic or even antiestrogenic effects occurring in such tissues.[3][9][7][19] Based on its mixed effects in different tissues, estetrol has been described as a unique, "natural" selective estrogen receptor modulator (SERM) rather than as a "weak estrogen."[3][10][19]
Estetrol is weakly estrogenic in breast/mammary gland, but shows very low potency in this tissue and, when administered in combination with estradiol, antagonizes the effects of estradiol.[3][19] Relative to estradiol, estetrol shows 100-fold lower potency in stimulation of the proliferation of human breast epithelial cells in vitro and of mouse mammary gland cells in vivo.[9] In animal models, estetrol shows antiestrogenic effects in mammary gland tissue comparable to those of the SERM tamoxifen and of ovariectomy, antagonizing the stimulatory effects of estradiol and preventing tumor development in a 7,12-dimethylbenz(a)anthracene (DMBA) mammary tumor model.[3][19][20] As such, it is anticipated that estetrol may cause minimal proliferation of breast tissue and that it may be useful in the treatment of breast cancer.[3][9]
Estetrol has relatively minimal effects on liver function.[9][7] In contrast to estradiol and ethinylestradiol, estetrol does not stimulate the hepatic production of SHBG in vitro.[6] In addition, it has been found to produce minimal changes in liver protein synthesis in women relative to ethinylestradiol, including production of sex hormone-binding globulin (SHBG), corticosteroid-binding globulin (CBG), angiotensinogen (AGT), ceruloplasmin, cholesterol, triglycerides, estrogen-sensitive coagulation proteins, insulin-like growth factor 1 (IGF-1), and insulin-like growth factor-binding proteins (IGFBPs).[9][3][7] In a clinical study, 10 mg/day estetrol showed only 15 to 20% of the increase of 20 μg/day ethinylestradiol on SHBG and AGT levels (both dosages being oral contraceptive dosages).[21][22] For comparison, it has been reported that a dosage of estradiol that is of similar potency to ethinylestradiol in terms of FSH suppression and hot flash relief possesses about 25% of the potency of ethinylestradiol on SHBG increase and about 35% of the potency of ethinylestradiol on AGT increase.[23] Estetrol has shown only a minor effect on hemostatic biomarkers, including both on coagulation and fibrinolysis.[7] Due to its minimal influence on liver function, estetrol may have a lower risk of venous thromboembolism (VTE) and other undesirable side effects.[3] This is notable, as VTE is a serious adverse effect of all known estrogens as well as synthetic SERMs (e.g., tamoxifen).[3] Also, oral estrogens like ethinylestradiol are associated with a reduction in lean body mass due to suppression of hepatic IGF-1 production, and this may not be expected with estetrol.[23][7]
Estetrol has potent estrogenic effects in the brain in terms of relief of hot flashes, antigonadotropic effects, and ovulation inhibition.[3] However, animal studies on the effects of estetrol on levels of allopregnanolone and β-endorphin in various brain areas have shown weak estrogenic effects when given alone and antiestrogenic effects in the presence of estradiol, suggesting that estetrol may have SERM-like effects in some regions of the brain.[19][24][25] Estetrol has mixed effects in the vascular system similarly.[19][26] It has been found to have estrogenic effects on atheroma prevention in arteries (and hence might be expected to have beneficial effects on atherosclerosis), but has antiestrogenic effects against estradiol-induced endothelial nitric oxide synthase activation and acceleration of endothelial healing.[19][26]
| Estrogen | Type | HF | VE | UCa | FSH | LH | HDL-C | SHBG | CBG | AGT | Liver |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol | Bioidentical | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Estrone | Bioidentical | ? | ? | ? | 0.3 | 0.3 | ? | ? | ? | ? | ? |
| Estriol | Bioidentical | 0.3 | 0.3 | 0.1 | 0.3 | 0.3 | 0.2 | ? | ? | ? | 0.67 |
| Estrone sulfate | Bioidentical | ? | 0.9 | 0.9 | 0.8–0.9 | 0.9 | 0.5 | 0.9 | 0.5–0.7 | 1.4–1.5 | 0.56–1.7 |
| Conjugated estrogens | Natural | 1.2 | 1.5 | 2.0 | 1.1–1.3 | 1.0 | 1.5 | 3.0–3.2 | 1.3–1.5 | 5.0 | 1.3–4.5 |
| Equilin sulfate | Natural | ? | ? | 1.0 | ? | ? | 6.0 | 7.5 | 6.0 | 7.5 | ? |
| Ethinylestradiol | Synthetic | 120 | 150 | 400 | 60–150 | 100 | 400 | 500–600 | 500–600 | 350 | 2.9–5.0 |
| Diethylstilbestrol | Synthetic | ? | ? | ? | 2.9–3.4 | ? | ? | 26–28 | 25–37 | 20 | 5.7–7.5 |
| Notes: Values are ratios, with estradiol as standard (i.e., 1.0). Abbreviations: HF = Clinical relief of hot flashes. VE = Increased proliferation of vaginal epithelium. UCa = Decrease in UCa. FSH = Suppression of FSH levels. LH = Suppression of LH levels. HDL-C, SHBG, CBG, and AGT = Increase in the serum levels of these liver proteins. Liver = Ratio of liver estrogenic effects to general/systemic estrogenic effects (specifically hot flashes relief and gonadotropin suppression). Type: Bioidentical = Identical to those found in humans. Natural = Naturally occurring but not identical to those found in humans (e.g., estrogens of other species). Synthetic = Man-made, does not occur naturally in animals or in the environment. Sources: See template. | |||||||||||
Antigonadotropic effects
Administration of single doses of estetrol to postmenopausal women strongly suppressed secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), demonstrating potent antigonadotropic effects.[3][5] Levels of LH were not suppressed by a dose of 0.1 or 1 mg, were slightly suppressed by a dose of 10 mg, and were profoundly suppressed by a dose of 100 mg (by a maximum of 48% 4-hours post-dose).[3][5] A profound and sustained inhibition of FSH levels (by a maximum of 41% 48-hours post-dose), lasting up to a week, was found with a 100 mg dose of estetrol (other doses were not assessed).[3][5] The antigonadotropic effects of estetrol result in inhibition of ovulation and hence are involved in its hormonal contraceptive effects in women.[3][27][5] In addition, the antigonadotropic effects of estetrol cause suppression of gonadal sex hormone production.[8] In healthy men, 40 mg/day estetrol suppressed total testosterone levels by 60% and estradiol levels by 62% when measured by day 28 of administration.[8] In another study of healthy men, testosterone levels were suppressed with estetrol therapy by 28% at 20 mg/day, by 60% at 40 mg/day, and by 76% at 60 mg/day after 4 weeks.[28] Suppression of testosterone levels is the primary basis for the use of estetrol in the treatment of prostate cancer.[8][28]
Pharmacokinetics
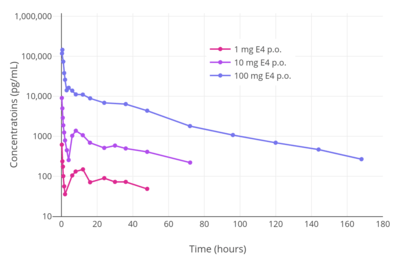
Estetrol shows high oral bioavailability.[5] Its oral bioavailability in rats was 70% relative to subcutaneous injection.[3] The high oral bioavailability of estetrol is in contrast to estradiol, estrone, and estriol (all very low, in the range of 5% and below), but is more similar to ethinylestradiol (38–48%).[5][23] Estetrol shows a high and linear dose–response relationship across oral doses of 0.1 to 100 mg in humans, and shows low interindividual variability.[3][5] Upon oral administration, estetrol is very rapidly absorbed, with maximal levels occurring within 15 to 80 minutes.[5][7] At a dosage of 20 mg/day estetrol, peak levels of estetrol of 3,490 pg/mL and trough levels of 2,005 pg/mL have been reported.[7] The high water solubility of estetrol makes it optimal for passage of the blood–brain barrier, and the drug may be expected to have effects in the central nervous system.[3] In accordance, estetrol shows clear central effects such as alleviation of hot flashes and antigonadotropic effects in humans.[14][27][8] In terms of plasma protein binding, estetrol is bound moderately to albumin, and is not bound to SHBG.[5][6] This is in contrast to estradiol, which binds to SHBG with high affinity, but is similar to estriol and ethinylestradiol, which have only very low affinity for SHBG.[5][3] Due to its lack of affinity for SHBG, the plasma distribution or availability for target tissues of estetrol is not limited or otherwise influenced by SHBG.[4]
Estetrol is metabolized slowly and minimally, and is not transformed into other estrogens such as estradiol, estrone, or estriol.[3][18][5] This is related to the fact that estetrol is already an end-stage product of phase I estrogen metabolism in humans.[5] The medication is conjugated via glucuronidation and to a lesser extent via sulfation.[3][7] The biological half-life of estetrol is about 28 hours, with a range of 18 to 60 hours.[5][7] The blood half-lives of estradiol and estriol, at about 1 to 2 hours and 20 minutes, respectively, are far shorter than that of estetrol, whereas the biological half-life of ethinylestradiol, at approximately 20 hours, is more similar to that of estetrol.[5] Enterohepatic recirculation may occur with estetrol, similarly to other steroidal estrogens, although it has also been reported that estetrol does not seem to enter the enterohepatic circulation.[5][17] Estetrol is excreted mostly or completely in urine, virtually entirely in the form of conjugates (unconjugated accounting for 0.2–0.7%).[7][3] In one study, a median of 79.7% (range 61.1–99.0%) was recovered from urine; this was primarily as estetrol glucuronide (median 60.7%, range 47.6–77.2%), and, to a lesser extent, as estetrol sulfate (median 17.6%, range 13.2–22.1%).[7]
Chemistry
Structures of major endogenous estrogens
|
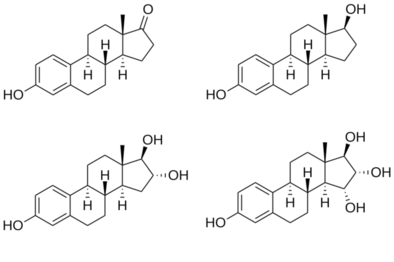
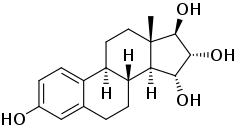
Estetrol, also known as 15α-hydroxyestriol or as estra-1,3,5(10)-triene-3,15α,16α,17β-tetrol, is a naturally occurring estrane steroid and a derivative of estrin (estra-1,3,5(10)-triene).[3][4] It has four hydroxyl groups, which is the basis for its abbreviation of E4.[3][4] For comparison, estriol (E3) has three hydroxyl groups, estradiol (E2) has two hydroxyl groups, and estrone (E1) has one hydroxyl group and one ketone.[3]
Synthesis
Chemical syntheses of estetrol have been published.[29]
History
Estetrol was discovered in 1965 by Egon Diczfalusy and coworkers at the Karolinska Institute in Stockholm, Sweden, via isolation from the urine of pregnant women.[3][11] Basic research on estetrol was conducted from 1965 to 1984.[3][4] It was established that estetrol is exclusively synthesized in the fetal liver and hence that it is a fetal estrogen.[3][4] By 1984, estetrol was regarded as a weak estrogen; interest fell, and research was virtually abandoned.[3][4] Subsequently, a project at Pantarhei Bioscience was started to investigate estetrol with state-of-the-art technologies in 2001, with the sole reasoning that estetrol must have some biological role or function of importance as it would not be produced in such high quantities in the fetus otherwise.[3] By 2008, estetrol was of major interest for potential clinical use, and development was in-progress.[3][4] As of 2018, it is in mid- to late-stage clinical development for a variety of indications.[1][2] It was initially developed by Pantarhei Bioscience and Estetra SA (subsequently renamed Estetra SPRL), and is now being developed by Mithra Pharmaceuticals.[1][2]
Society and culture
Brand names
Estetrol has the tentative brand names Donesta as a single-drug formulation and Estelle in combination with drospirenone.[1][2]
Availability
Estetrol is still in clinical trials and is not yet marketed in any country.[1][2]
See also
References
- "Estetrol - Mithra Pharmaceuticals - AdisInsight".
- "Drospirenone/estetrol - Mithra Pharmaceuticals - AdisInsight".
- Coelingh Bennink HJ, Holinka CF, Diczfalusy E (2008). "Estetrol review: profile and potential clinical applications". Climacteric. 11 Suppl 1: 47–58. doi:10.1080/13697130802073425. PMID 18464023.
- Visser M, Coelingh Bennink HJ (March 2009). "Clinical applications for estetrol" (PDF). J. Steroid Biochem. Mol. Biol. 114 (1–2): 85–9. doi:10.1016/j.jsbmb.2008.12.013. PMID 19167495.
- Visser M, Holinka CF, Coelingh Bennink HJ (2008). "First human exposure to exogenous single-dose oral estetrol in early postmenopausal women". Climacteric. 11 Suppl 1: 31–40. doi:10.1080/13697130802056511. PMID 18464021.
- Hammond GL, Hogeveen KN, Visser M, Coelingh Bennink HJ (2008). "Estetrol does not bind sex hormone binding globulin or increase its production by human HepG2 cells". Climacteric. 11 Suppl 1: 41–6. doi:10.1080/13697130701851814. PMID 18464022.
- Mawet M, Maillard C, Klipping C, Zimmerman Y, Foidart JM, Coelingh Bennink HJ (2015). "Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives". Eur J Contracept Reprod Health Care. 20 (6): 463–75. doi:10.3109/13625187.2015.1068934 (inactive 2019-12-13). PMC 4699469. PMID 26212489.
- Dutman, E.; Zimmerman, Y.; Coelingh-Bennink, H. (2017). "The effects of the human fetal estrogen estetrol (E4) in healthy men to estimate its potential use for the treatment of prostate cancer". European Urology Supplements. 16 (3): e362–e364. doi:10.1016/S1569-9056(17)30276-2. ISSN 1569-9056.
- Gérard C, Blacher S, Communal L, Courtin A, Tskitishvili E, Mestdagt M, Munaut C, Noel A, Gompel A, Péqueux C, Foidart JM (January 2015). "Estetrol is a weak estrogen antagonizing estradiol-dependent mammary gland proliferation". J. Endocrinol. 224 (1): 85–95. doi:10.1530/JOE-14-0549. PMID 25359896.
- Nath A, Sitruk-Ware R (June 2009). "Pharmacology and clinical applications of selective estrogen receptor modulators". Climacteric. 12 (3): 188–205. doi:10.1080/13697130802657896. PMID 19387883.
- Hagen AA, Barr M, Diczfalusy E (June 1965). "Metabolism of 17-beta-oestradiol-4-14-C in early infancy". Acta Endocrinol. 49 (2): 207–20. doi:10.1530/acta.0.0490207. PMID 14303250.
- Rapkin AJ, Winer SA (May 2007). "Drospirenone: a novel progestin". Expert Opin Pharmacother. 8 (7): 989–99. doi:10.1517/14656566.8.7.989. PMID 17472544.
- Oelkers W (March 2004). "Drospirenone, a progestogen with antimineralocorticoid properties: a short review". Mol. Cell. Endocrinol. 217 (1–2): 255–61. doi:10.1016/j.mce.2003.10.030. PMID 15134826.
- Coelingh Bennink HJ, Verhoeven C, Zimmerman Y, Visser M, Foidart JM, Gemzell-Danielsson K (September 2016). "Clinical effects of the fetal estrogen estetrol in a multiple-rising-dose study in postmenopausal women". Maturitas. 91: 93–100. doi:10.1016/j.maturitas.2016.06.017. PMID 27451327.
- Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 989–. ISBN 978-0-7817-1750-2.
- Coelingh Bennink F, Holinka CF, Visser M, Coelingh Bennink HJ (2008). "Maternal and fetal estetrol levels during pregnancy". Climacteric. 11 Suppl 1: 69–72. doi:10.1080/13697130802056321. PMID 18464026.
- Holinka CF, Diczfalusy E, Coelingh Bennink HJ (May 2008). "Estetrol: a unique steroid in human pregnancy". J. Steroid Biochem. Mol. Biol. 110 (1–2): 138–43. doi:10.1016/j.jsbmb.2008.03.027. PMID 18462934.
- Visser M, Foidart JM, Coelingh Bennink HJ (2008). "In vitro effects of estetrol on receptor binding, drug targets and human liver cell metabolism". Climacteric. 11 Suppl 1: 64–8. doi:10.1080/13697130802050340. PMID 18464025.
- Gérard C, Mestdagt M, Tskitishvili E, Communal L, Gompel A, Silva E, Arnal JF, Lenfant F, Noel A, Foidart JM, Péqueux C (July 2015). "Combined estrogenic and anti-estrogenic properties of estetrol on breast cancer may provide a safe therapeutic window for the treatment of menopausal symptoms". Oncotarget. 6 (19): 17621–36. doi:10.18632/oncotarget.4184. PMC 4627333. PMID 26056044.
- Visser M, Kloosterboer HJ, Bennink HJ (April 2012). "Estetrol prevents and suppresses mammary tumors induced by DMBA in a rat model". Horm Mol Biol Clin Investig. 9 (1): 95–103. doi:10.1515/hmbci-2012-0015. PMID 25961355.
- Farris M, Bastianelli C, Rosato E, Brosens I, Benagiano G (October 2017). "Pharmacodynamics of combined estrogen-progestin oral contraceptives: 2. effects on hemostasis". Expert Rev Clin Pharmacol. 10 (10): 1129–1144. doi:10.1080/17512433.2017.1356718. PMID 28712325.
- Kluft C, Zimmerman Y, Mawet M, Klipping C, Duijkers IJ, Neuteboom J, Foidart JM, Bennink HC (February 2017). "Reduced hemostatic effects with drospirenone-based oral contraceptives containing estetrol vs. ethinyl estradiol". Contraception. 95 (2): 140–147. doi:10.1016/j.contraception.2016.08.018. PMID 27593335.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Pluchino N, Santoro AN, Casarosa E, Giannini A, Genazzani A, Russo M, Russo N, Petignat P, Genazzani AR (September 2014). "Effect of estetrol administration on brain and serum allopregnanolone in intact and ovariectomized rats". J. Steroid Biochem. Mol. Biol. 143: 285–90. doi:10.1016/j.jsbmb.2014.04.011. PMID 24787659.
- Pluchino N, Drakopoulos P, Casarosa E, Freschi L, Petignat P, Yaron M, Genazzani AR (March 2015). "Effect of estetrol on Beta-Endorphin level in female rats". Steroids. 95: 104–10. doi:10.1016/j.steroids.2015.01.003. PMID 25595451.
- Abot A, Fontaine C, Buscato M, Solinhac R, Flouriot G, Fabre A, Drougard A, Rajan S, Laine M, Milon A, Muller I, Henrion D, Adlanmerini M, Valéra MC, Gompel A, Gerard C, Péqueux C, Mestdagt M, Raymond-Letron I, Knauf C, Ferriere F, Valet P, Gourdy P, Katzenellenbogen BS, Katzenellenbogen JA, Lenfant F, Greene GL, Foidart JM, Arnal JF (October 2014). "The uterine and vascular actions of estetrol delineate a distinctive profile of estrogen receptor α modulation, uncoupling nuclear and membrane activation". EMBO Mol Med. 6 (10): 1328–46. doi:10.15252/emmm.201404112. PMC 4287935. PMID 25214462.
- Duijkers IJ, Klipping C, Zimmerman Y, Appels N, Jost M, Maillard C, Mawet M, Foidart JM, Coelingh Bennink HJ (2015). "Inhibition of ovulation by administration of estetrol in combination with drospirenone or levonorgestrel: Results of a phase II dose-finding pilot study". Eur J Contracept Reprod Health Care. 20 (6): 476–89. doi:10.3109/13625187.2015.1074675 (inactive 2019-12-13). PMC 4673580. PMID 26394847.
- Coelingh Bennink HJ, Zimmerman Y, Verhoeven C, Dutman AE, Mensinga T, Kluft C, Reisman Y, Debruyne FM (June 2018). "A Dose Escalating Study with the Fetal Estrogen Estetrol in Healthy Males". J. Clin. Endocrinol. Metab. 103 (9): 3239–3249. doi:10.1210/jc.2018-00147. PMID 29931320.
- Warmerdam EG, Visser M, Coelingh Bennink HJ, Groen M (2008). "A new route of synthesis of estetrol". Climacteric. 11 Suppl 1: 59–63. doi:10.1080/13697130802054078. PMID 18464024.
- http://apps.who.int/medicinedocs/documents/s23133en/s23133en.pdf
Further reading
- Sakamoto H, Ohtani K, Satoh K (March 1995). "[Estetrol (E4)]". Nippon Rinsho (in Japanese). 53 Su Pt 2: 566–8. PMID 8753305.
- Holinka CF, Diczfalusy E, Coelingh Bennink HJ (May 2008). "Estetrol: a unique steroid in human pregnancy". J. Steroid Biochem. Mol. Biol. 110 (1–2): 138–43. doi:10.1016/j.jsbmb.2008.03.027. PMID 18462934.
- Coelingh Bennink HJ, Holinka CF, Diczfalusy E (2008). "Estetrol review: profile and potential clinical applications". Climacteric. 11 Suppl 1: 47–58. doi:10.1080/13697130802073425. PMID 18464023.
- Warmerdam EG, Visser M, Coelingh Bennink HJ, Groen M (2008). "A new route of synthesis of estetrol". Climacteric. 11 Suppl 1: 59–63. doi:10.1080/13697130802054078. PMID 18464024.
- Visser M, Foidart JM, Coelingh Bennink HJ (2008). "In vitro effects of estetrol on receptor binding, drug targets and human liver cell metabolism". Climacteric. 11 Suppl 1: 64–8. doi:10.1080/13697130802050340. PMID 18464025.
- Coelingh Bennink F, Holinka CF, Visser M, Coelingh Bennink HJ (2008). "Maternal and fetal estetrol levels during pregnancy". Climacteric. 11 Suppl 1: 69–72. doi:10.1080/13697130802056321. PMID 18464026.
- Visser M, Coelingh Bennink HJ (March 2009). "Clinical applications for estetrol" (PDF). J. Steroid Biochem. Mol. Biol. 114 (1–2): 85–9. doi:10.1016/j.jsbmb.2008.12.013. PMID 19167495.
- Krøll J (April 2014). "Estetrol, molecular chaperones, and the epigenetics of longevity and cancer resistance". Rejuvenation Res. 17 (2): 157–8. doi:10.1089/rej.2013.1483. PMID 23992378.
External links
- Estetrol (Donesta, E4) - AdisInsight
- Estetrol/Drospirenone (Estelle, E4/DRSP) - AdisInsight
- Estetrol official website - Mithra Pharmaceuticals