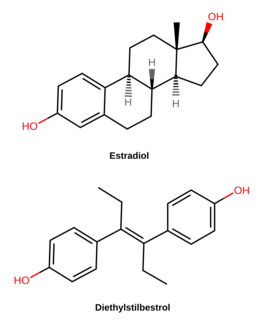
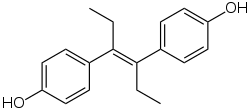
Diethylstilbestrol
Diethylstilbestrol (DES), also known as stilbestrol or stilboestrol, is a nonsteroidal estrogen medication, which is rarely used.[4][5] In the past, it was widely used for a variety of indications, including pregnancy support for women with a history of recurrent miscarriage, hormone therapy for menopausal symptoms and estrogen deficiency in women, treatment of prostate cancer in men and breast cancer in women, and other uses. Today, it is only used in the treatment of prostate cancer and less commonly breast cancer.[6] While most commonly taken by mouth, DES was available for use by other routes as well, for instance, vaginal, topical, and by injection.
 | |
 | |
| Clinical data | |
|---|---|
| Other names | DES; Stilboestrol; Stilbestrol; (E)-11,12-Diethyl-4,13-stilbenediol |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | By mouth, vaginal, topical, intravenous, intramuscular injection (as an ester) |
| Drug class | Nonsteroidal estrogen |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | Well-absorbed[1] |
| Protein binding | >95%[2] |
| Metabolism | Hydroxylation, oxidation, glucuronidation[1][2][3] |
| Metabolites | • (Z,Z)-Dienestrol[1] • Paroxypropione[1] • Glucuronides[2][3] |
| Elimination half-life | 24 hours[1] |
| Excretion | Urine, feces[2][3] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.253 |
| Chemical and physical data | |
| Formula | C18H20O2 |
| Molar mass | 268.356 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
DES is an estrogen, or an agonist of the estrogen receptors, the biological target of estrogens like estradiol.[5] It is a synthetic and nonsteroidal estrogen of the stilbestrol group, and differs from the natural estrogen estradiol in various ways.[5] Compared to estradiol, DES has greatly improved bioavailability when taken by mouth, is more resistant to metabolism, and shows relatively increased effects in certain parts of the body like the liver and uterus.[5] These differences result in DES having an increased risk of blood clots, cardiovascular issues, and certain other adverse effects.[5]
DES was discovered in 1938.[7] From about 1940 to 1971, the medication was given to pregnant women in the incorrect belief that it would reduce the risk of pregnancy complications and losses.[7] In 1971, DES was shown to cause clear-cell carcinoma, a rare vaginal tumor, in girls and women who had been exposed to this medication in utero.[7] The United States Food and Drug Administration subsequently withdrew approval of DES as a treatment for pregnant women.[7] Follow-up studies have indicated that DES also has the potential to cause a variety of significant adverse medical complications during the lifetimes of those exposed.[7][8]
The United States National Cancer Institute recommends[9] women born to mothers who took DES to undergo special medical exams on a regular basis to screen for complications as a result of the medication. Individuals who were exposed to DES during their mothers' pregnancies are commonly referred to as "DES daughters" and "DES sons".[7][10] Since the discovery of the toxic effects of DES, it has largely been discontinued and is now mostly no longer marketed.[7][11]
Medical uses
DES has been used in the past for the following indications:[12]
- Recurrent miscarriage in pregnancy
- Menopausal hormone therapy for the treatment of menopausal symptoms such as hot flashes and vaginal atrophy
- Hormone therapy for hypoestrogenism (e.g., gonadal dysgenesis, premature ovarian failure, and after oophorectomy)
- Postpartum lactation suppression to prevent or reverse breast engorgement[13]
- Gonorrheal vaginitis (discontinued following the introduction of the antibiotic penicillin)
- Prostate cancer and breast cancer
- Prevention of tall stature in tall adolescent girls
- As an emergency postcoital contraceptive
- As a means of chemical castration for hypersexuality and paraphilias in men and sex offenders
- Prevention of the testosterone flare at the start of gonadotropin-releasing hormone agonist (GnRH agonist) therapy[14][15][16][17][18][19][20]
DES was used at a dosage of 0.2 to 0.5 mg/day in menopausal hormone therapy.[21]
Interest in the use of DES to treat prostate cancer in men continues today.[22][23][24][25][26][27][28] However, some researchers have advocated for the use of bioidentical parenteral estrogens like polyestradiol phosphate in favor of oral synthetic estrogens like DES due to their much lower risk of cardiovascular toxicity.[29][26][28] In addition to prostate cancer, some interest in the use of DES to treat breast cancer in women continues today as well.[30][31] However, similarly to the case of prostate cancer, some researchers have argued for the use bioidentical estrogens like estradiol instead of DES for breast cancer.[30][32]
| Route/form | Estrogen | Dosage | |
|---|---|---|---|
| Oral | Estradiol | 10 mg 3x/day AI-resistant: 2 mg 1–3x/day | |
| Estradiol valerate | AI-resistant: 2 mg 1–3x/day | ||
| Conjugated estrogens | 10 mg 3x/day | ||
| Ethinylestradiol | 0.5–1 mg 3x/day | ||
| Diethylstilbestrol | 5 mg 3x/day | ||
| Dienestrol | 5 mg 3x/day | ||
| IM or SC injection | Estradiol benzoate | 5 mg 2–3x/week | |
| Estradiol dipropionate | 5 mg 2–3x/week | ||
| Estradiol valerate | 30 mg 1x/2 weeks | ||
| Polyestradiol phosphate | 40–80 mg 1x/4 weeks | ||
| Estrone | 5 mg ≥3x/week | ||
| Notes: (1) Only in women who are at least 5 years postmenopausal. (2) Dosages are not necessarily equivalent. Sources: See template. | |||
| Route/form | Estrogen | Dosage | |
|---|---|---|---|
| Oral | Estradiol | 1–2 mg 3x/day | |
| Conjugated estrogens | 1.25–2.5 mg 3x/day | ||
| Ethinylestradiol | 0.15–3 mg/day | ||
| Ethinylestradiol sulfonate | 1–2 mg 1x/week | ||
| Diethylstilbestrol | 1–3 mg/day | ||
| Dienestrol | 5 mg/day | ||
| Hexestrol | 5 mg/day | ||
| Fosfestrol | 100–480 mg 1–3x/day | ||
| Chlorotrianisene | 12–48 mg/day | ||
| Quadrosilan | 900 mg/day | ||
| Estramustine phosphate | 140–1400 mg/day | ||
| Transdermal patch | Estradiol | 2–6x 100 μg/day Scrotal: 1x 100 μg/day | |
| IM or SC injection | Estradiol benzoate | 1.66 mg 3x/week | |
| Estradiol dipropionate | 5 mg 1x/week | ||
| Estradiol valerate | 10–40 mg 1x/1–2 weeks | ||
| Estradiol undecylate | 100 mg 1x/4 weeks | ||
| Polyestradiol phosphate | Alone: 160–320 mg 1x/4 weeks With oral EE: 40–80 mg 1x/4 weeks | ||
| Estrone | 2–4 mg 2–3x/week | ||
| IV injection | Fosfestrol | 300–1200 mg 1–7x/week | |
| Estramustine phosphate | 240–450 mg/day | ||
| Note: Dosages are not necessarily equivalent. Sources: See template. | |||
Side effects
At doses above 1 mg/day by mouth, DES is associated with high rates of side effects including nausea, vomiting, abdominal discomfort, headache, and bloating, with an incidence of 15 to 50%.[33]
In studies of DES as a form of high-dose estrogen therapy for men with prostate cancer, it has been associated with considerable cardiovascular morbidity and mortality.[23] The risk is dose-dependent.[23] A dosage of 5 mg/day DES has been associated with a 36% increase in non-cancer-related (mostly cardiovascular) deaths.[23] In addition, there is an up to 15% incidence of venous thromboembolism.[34] A 3 mg/day dosage of DES has been associated with an incidence of thromboembolism of 9.6 to 17%, with an incidence of cardiovascular complications of 33.3%.[23] A lower dosage of 1 mg/day DES has been associated with a rate of death due to cardiovascular events of 14.8% (relative to 8.3% for orchiectomy alone).[23]
In men treated with it for prostate cancer, DES has been found to produce high rates of gynecomastia (breast development) of 41 to 77%.[35]
The pigmentation of the breast areolae are often very dark and almost black with DES therapy.[36][37][38][39][40][41] The pigmentation that occurs with synthetic estrogens such as DES is much greater than with natural estrogens such as estradiol.[36]
Long-term effects
DES has been linked to a variety of long-term adverse effects, such as increased risk of vaginal clear-cell adenocarcinoma, vaginal adenosis, T-shaped uterus, uterine fibroids, cervical weakness, breast cancer, infertility, hypogonadism, intersex defects, depression, and others, in women who were treated with it during pregnancy and/or in their offspring.[42]
A comprehensive animal study in 1993 found a plethora of adverse effects from DES such as [but not limited to] genotoxicity (due to quinone metabolite), teratogenicity, penile and testicular hypoplasia, cryptorchidism (rats and rhesus monkeys), liver and renal cancer (hamsters), ovarian papillary carcinoma (canines), and malignant uterine mesothelioma (squirrel monkeys). [43] Evidence was also found linking ADHD to F2 generations, demonstrating that there is at least some neurological and transgenerational effects in addition to the carcinogenic. [44] Rodent studies reveal female reproductive tract cancers and abnormalities reaching to the F2 generation, and there is evidence of adverse effects such as irregular menstrual cycles in grandchildren of DES mothers. [45] Additionally, evidence also points to transgenerational effects in F2 sons like hypospadias. [46] At this time however, the extent of DES transgenerational effects in regards to humans is not fully understood.
Overdose
DES has been assessed in the past in clinical studies at extremely high dosages of as much as 1,500 mg/day.[30][47]
Pharmacology
Pharmacodynamics
Estrogenic activity
DES is an estrogen; specifically, it is a highly potent full agonist of both of the estrogen receptors (ERs).[48][49] It has approximately 468% and 295% of the affinity of estradiol at the ERα and ERβ, respectively.[50] However, EC50 values of 0.18 nM and 0.06 nM of DES for the ERα and ERβ, respectively, have been reported, suggesting, in spite of its binding affinity for the two receptors, several-fold preference for activation of the ERβ over the ERα.[51]
A dosage of 1 mg/day DES is approximately equivalent to a dosage of 50 µg/day ethinylestradiol in terms of systemic estrogenic potency.[1] Similarly to ethinylestradiol, DES shows a marked and disproportionately strong effect on liver protein synthesis.[5] Whereas its systemic estrogenic potency was about 3.8-fold of that of estropipate (piperazine estrone sulfate), which has similar potency to micronized estradiol, the hepatic estrogenic potency of DES was 28.4-fold that of estropipate (or about 7.5-fold stronger potency for a dosage with equivalent systemic estrogenic effect).[1]
DES has at least three mechanisms of action in the treatment of prostate cancer in men.[52] It suppresses gonadal androgen production and hence circulating androgen levels due to its antigonadotropic effects; it stimulates hepatic sex hormone-binding globulin (SHBG) production, thereby increasing circulating levels of SHBG and decreasing the free fraction of testosterone and dihydrotestosterone (DHT) in the circulation; and it may have direct cytotoxic effects in the testes and prostate gland.[52] DES has also been found to decrease DNA synthesis at high doses.[52]
DES is a long-acting estrogen, with a nuclear retention of around 24 hours.[53][54]
| Estrogen | Type | HF | VE | UCa | FSH | LH | HDL-C | SHBG | CBG | AGT | Liver |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol | Bioidentical | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Estrone | Bioidentical | ? | ? | ? | 0.3 | 0.3 | ? | ? | ? | ? | ? |
| Estriol | Bioidentical | 0.3 | 0.3 | 0.1 | 0.3 | 0.3 | 0.2 | ? | ? | ? | 0.67 |
| Estrone sulfate | Bioidentical | ? | 0.9 | 0.9 | 0.8–0.9 | 0.9 | 0.5 | 0.9 | 0.5–0.7 | 1.4–1.5 | 0.56–1.7 |
| Conjugated estrogens | Natural | 1.2 | 1.5 | 2.0 | 1.1–1.3 | 1.0 | 1.5 | 3.0–3.2 | 1.3–1.5 | 5.0 | 1.3–4.5 |
| Equilin sulfate | Natural | ? | ? | 1.0 | ? | ? | 6.0 | 7.5 | 6.0 | 7.5 | ? |
| Ethinylestradiol | Synthetic | 120 | 150 | 400 | 60–150 | 100 | 400 | 500–600 | 500–600 | 350 | 2.9–5.0 |
| Diethylstilbestrol | Synthetic | ? | ? | ? | 2.9–3.4 | ? | ? | 26–28 | 25–37 | 20 | 5.7–7.5 |
| Notes: Values are ratios, with estradiol as standard (i.e., 1.0). Abbreviations: HF = Clinical relief of hot flashes. VE = Increased proliferation of vaginal epithelium. UCa = Decrease in UCa. FSH = Suppression of FSH levels. LH = Suppression of LH levels. HDL-C, SHBG, CBG, and AGT = Increase in the serum levels of these liver proteins. Liver = Ratio of liver estrogenic effects to general/systemic estrogenic effects (specifically hot flashes relief and gonadotropin suppression). Type: Bioidentical = Identical to those found in humans. Natural = Naturally occurring but not identical to those found in humans (e.g., estrogens of other species). Synthetic = Man-made, does not occur naturally in animals or in the environment. Sources: See template. | |||||||||||
| Estrogen | Type | Class | ETD (mg/14 days) | EPD (mg/14 days) | EPD (mg/day) | MSD (mg/14 days) | MSD (mg/day) | TSD (mg/day) |
|---|---|---|---|---|---|---|---|---|
| Estradiol (non-micronized) | Bioidentical | Steroidal | ? | ≥120–300 | ? | ? | ? | ? |
| Estradiol (micronized) | Bioidentical | Steroidal | ? | 60–80 | 4.3 | 14–28 | 1.0–2.0 | >8 |
| Estradiol valerate | Bioidentical | Steroidal | 6–10 | 60–80 | 4.3 | 14–28 | 1.0–2.0 | >8 |
| Estradiol benzoate | Bioidentical | Steroidal | ? | 60–140 | 4.5 | ? | ? | ? |
| Estriol | Bioidentical | Steroidal | 20a | 120–150b | 10.0–10.7b | 28–84 | 1.0–6.0 | ? |
| Estriol succinate | Bioidentical | Steroidal | ? | 140–150b | 10.0–10.7b | 28–84 | 2.0–6.0 | ? |
| Conjugated estrogens | Natural | Steroidal | 5–12 | 60–80 | 4.3 | 8.4–17.5 | 0.625–1.25 | 7.5 |
| Ethinylestradiol | Synthetic | Steroidal | 0.2 | 1.0–2.0 | 0.071–0.11 | 0.28 | 0.02–0.04 | 0.1 |
| Mestranol | Synthetic | Steroidal | 0.3 | 1.5–3.0 | 0.11–0.13 | 0.3–0.5 | 0.025 | ? |
| Quinestrol | Synthetic | Steroidal | 0.3 | 2.0–4.0 | 0.14–0.29 | ? | 0.025–0.05 | ? |
| Methylestradiol | Synthetic | Steroidal | ? | 2.0 | ? | ? | ? | ? |
| Diethylstilbestrol | Synthetic | Nonsteroidal | 2.5 | 20–30 | 1.4–2.1 | ? | 0.5–2.0 | 3 |
| Diethylstilbestrol dipropionate | Synthetic | Nonsteroidal | ? | 15–30 | 1.1–1.4 | ? | ? | ? |
| Dienestrol | Synthetic | Nonsteroidal | ? | 30 | ? | ? | 0.5–4.0 | ? |
| Dienestrol diacetate | Synthetic | Nonsteroidal | 3–5 | 30–60 | 2.9–4.3 | ? | ? | ? |
| Hexestrol | Synthetic | Nonsteroidal | ? | 70–110 | ? | ? | ? | ? |
| Hexestrol diacetate | Synthetic | Nonsteroidal | ? | 45 | ? | ? | ? | ? |
| Chlorotrianisene | Synthetic | Nonsteroidal | ? | >100 | ? | ? | ? | ? |
| Methallenestril | Synthetic | Nonsteroidal | ? | 400 | ? | ? | ? | ? |
| Note: The OID of EE is 0.1 mg/day. Footnotes: a = Very variable, often higher. b = In divided doses, 3x/day; irregular and atypical proliferation. Sources: See template. | ||||||||
| Estrogen | Form | Major brand name(s) | EPD (14 days) | Duration | |
|---|---|---|---|---|---|
| Diethylstilbestrol (DES) | Oil solution | ? | 20 mg | 3 mg ≈ 3 days | |
| Diethylstilbestrol dipropionate | Oil solution | Cyren B | 12.5–15 mg | 2.5 mg ≈ 5 days | |
| Aqueous suspension | ? | 5 mg | ? mg = 21–28 days | ||
| Dimestrol (DES dimethyl ether) | Oil solution | Depot-Cyren, Depot-Oestromon, Retalon Retard | 20–40 mg | ? | |
| Fosfestrol (DES diphosphate)a | Aqueous solution | Honvan | ? | <1 day | |
| Dienestrol diacetate | Aqueous suspension | Farmacyrol-Kristallsuspension | 50 mg | ? | |
| Hexestrol dipropionate | Oil solution | Hormoestrol, Retalon Oleosum | 25 mg | ? | |
| Hexestrol diphosphatea | Aqueous solution | Cytostesin, Pharmestrin, Retalon Aquosum | ? | Very short | |
| Note: All by intramuscular injection unless otherwise noted. Footnotes: a = By intravenous injection. Sources: See template. | |||||
Antigonadotropic effects
Due to its estrogenic activity, DES has antigonadotropic effects.[55][52][56][57] That is, it exerts negative feedback on the hypothalamic–pituitary–gonadal axis (HPG axis), suppresses the secretion of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and suppresses sex hormone production as well as gamete production or maturation in the gonads.[55][52][56][57] A study of ovulation inhibition in women found that 5 mg/day oral DES was 92% effective, with ovulation occurring in only a single cycle.[58][59] DES consistently suppresses testosterone levels in men into the castrate range (<50 ng/dL) within 1 to 2 weeks at doses of 3 mg/day and above.[55][57] Conversely, a dosage of 1 mg/day DES is unable to fully suppress testosterone levels into the castrate range in men, which instead often stabilize at just above castrate levels (>50 ng/dL).[23][52][56] However, it has also been reported that 1 mg/day DES results in approximately 50% suppression of testosterone levels, albeit with wide interindividual variability.[55] It has been said that doses of DES of less than 1 mg/day have no effect on testosterone levels.[55] However, the addition of an "extremely low" dosage of 0.1 mg/day DES to cyproterone acetate has been found to result in a synergistic antigonadotropic effect and to suppress testosterone levels into the castrate range in men.[60][61][62]
Other activities
In addition to the ERs, an in vitro study found that DES also possesses activity, albeit relatively weak, at a variety of other steroid hormone receptors.[51] Whereas the study found EC50 values of 0.18 nM and 0.06 nM of DES for the ERα and ERβ, respectively, the medication showed significant glucocorticoid activity at a concentration of 1 μM that surpassed that of 0.1 nM dexamethasone, as well as significant antagonism of the androgen, progesterone, and mineralocorticoid receptors (75%, 85%, and 50% inhibition of positive control stimulation, respectively, all at a concentration of 1 μM).[51] It also showed approximately 25% inhibition of the activation of PPARγ and LXRα at a concentration of 10 μM.[51] The researchers stated that, to the best of their knowledge, they were the first to report such actions of DES, and hypothesized that these actions could be involved in the clinical effects of DES, for instance, in prostate cancer (notably in which particularly high dosages of DES are employed).[51] However, they also noted that the importance of the activities requires further study in animal models at pharmacologically relevant doses.[51]
DES has been identified as an antagonist of all three isotypes of the estrogen-related receptors (ERRs), the ERRα, ERRβ, and ERRγ.[63]
Pharmacokinetics
DES is well-absorbed with oral administration.[1] With an oral dosage of 1 mg/day DES, plasma levels of DES at 20 hours following the last dose ranged between 0.9 and 1.9 ng/mL (3.4 to 7.1 nmol/L).[1] The distribution half-life of DES is 80 minutes.[1] It has no affinity for SHBG or corticosteroid-binding globulin, and hence is not bound to these proteins in the circulation.[64] The plasma protein binding of DES is greater than 95%.[2] Hydroxylation of the aromatic rings of DES and subsequent conjugation of the ethyl side chains accounts for 80 to 90% of DES metabolism, while oxidation accounts for the remaining 10 to 20% and is dominated by conjugation reactions.[2][3] Conjugation of DES consists of glucuronidation, while oxidation includes dehydrogenation into (Z,Z)-dienestrol.[1][2][3] The medication is also known to produce paroxypropione as a metabolite.[65] DES produces transient quinone-like reactive intermediates that cause cellular and genetic damage, which may explain the known carcinogenic effects of DES in humans.[1] However, other research suggests that the toxic effects of DES may simply be due to overactivation of the ERs.[66] The elimination half-life of DES is 24 hours.[1] The metabolites of DES are excreted in urine and feces.[2][3]
Sublingual administration of DES appears to have about the same estrogenic potency of oral DES in women.[67]
Intrauterine DES has been studied for the treatment of uterine hypoplasia.[68]
Chemistry
DES belongs to the stilbestrol (4,4'-dihydroxystilbene) group of compounds.[72] It is a nonsteroidal open-ring analogue of the steroidal estrogen estradiol.[69] DES can be prepared from anethole, which also happens to be weakly estrogenic.[72][73][71][70] Anethole was demethylated to form anol and anol then spontaneously dimerized into dianol and hexestrol, with DES subsequently being synthesized via structural modification of hexestrol.[72][73][71][70] As shown by X-ray crystallography, the molecular dimensions of DES are almost identical to those of estradiol, particularly in regards to the distance between the terminal hydroxyl groups.[70]
History
Synthesis
DES was first synthesized in early 1938 by Leon Golberg, then a graduate student of Sir Robert Robinson at the Dyson Perrins Laboratory at the University of Oxford. Golberg's research was based on work by Wilfrid Lawson at the Courtauld Institute of Biochemistry, (led by Sir Edward Charles Dodds at Middlesex Hospital Medical School now part of University College London). A report of its synthesis was published in Nature on 5 February 1938.[74][75][76]
DES research was funded by the UK Medical Research Council (MRC), which had a policy against patenting drugs discovered using public funds. Because it was not patented, DES was produced by more than 200 pharmaceutical and chemical companies worldwide.
Clinical use
DES (in tablets up to 5 mg) was approved by the United States Food and Drug Administration (FDA) on September 19, 1941 for four indications: gonorrheal vaginitis, atrophic vaginitis, menopausal symptoms, and postpartum lactation suppression to prevent breast engorgement.[76] The gonorrheal vaginitis indication was dropped when the antibiotic penicillin became available. From its very inception, the drug was highly controversial.[77][78]
In 1941, Charles Huggins and Clarence Hodges at the University of Chicago found estradiol benzoate and DES to be the first effective drugs for the treatment of metastatic prostate cancer.[79][80] DES was the first cancer drug.[81]
Orchiectomy or DES or both were the standard initial treatment for symptomatic advanced prostate cancer for over 40 years, until the GnRH agonist leuprorelin was found to have efficacy similar to DES without estrogenic effects and was approved in 1985.[82]
From the 1940s until the late 1980s, DES was FDA-approved as estrogen-replacement therapy for estrogen deficiency states such as ovarian dysgenesis, premature ovarian failure, and after oophorectomy.
In the 1940s, DES was used off-label to prevent adverse pregnancy outcomes in women with a history of miscarriage. On July 1, 1947, the FDA approved the use of DES for this indication. The first such approval was granted to Bristol-Myers Squibb, allowing use of 25 mg (and later 100 mg) tablets of DES during pregnancy. Approvals were granted to other pharmaceutical companies later in the same year.[83] The recommended regimen started at 5 mg per day in the seventh and eighth weeks of pregnancy (from first day of last menstrual period), increased every other week by 5 mg per day through the 14th week, and then increased every week by 5 mg per day from 25 mg per day in the 15th week to 125 mg per day in the 35th week of pregnancy.[84] DES was originally considered effective and safe for both the pregnant woman and the developing baby. It was aggressively marketed and routinely prescribed. Sales peaked in 1953.
In the early 1950s, a double-blind clinical trial at the University of Chicago assessed pregnancy outcomes in women who were assigned to either receive or not receive DES.[85] The study showed no benefit of taking DES during pregnancy; adverse pregnancy outcomes were not reduced in the women who were given DES. By the late 1960s, six of seven leading textbooks of obstetrics said DES was ineffective at preventing miscarriage.[83][86]
Despite an absence of evidence supporting the use of DES to prevent adverse pregnancy outcomes, DES continued to be given to pregnant women through the 1960s. In 1971, a report published in the New England Journal of Medicine showed a probable link between DES and vaginal clear cell adenocarcinoma in girls and young women who had been exposed to this drug in utero. Later in the same year, the FDA sent an FDA Drug Bulletin to all U.S. physicians advising against the use of DES in pregnant women. The FDA also removed prevention of miscarriage as an indication for DES use and added pregnancy as a contraindication for DES use.[87] On February 5, 1975, the FDA ordered 25 mg and 100 mg tablets of DES withdrawn, effective February 18, 1975.[88] The number of persons exposed to DES during pregnancy or in utero during 1940–1971 is unknown, but may be as high as 2 million in the United States. DES was also used in other countries, most notably France, the Netherlands, and Great Britain.
From the 1950s through the beginning of the 1970s, DES was prescribed to prepubescent girls to begin puberty and thus stop growth by closing growth plates in the bones. Despite its clear link to cancer, doctors continued to recommend the hormone for "excess height".[89]
In 1960, DES was found to be more effective than androgens in the treatment of advanced breast cancer in postmenopausal women.[90] DES was the hormonal treatment of choice for advanced breast cancer in postmenopausal women until 1977, when the FDA approved tamoxifen, a selective estrogen receptor modulator with efficacy similar to DES but fewer side effects.[91]
Several sources from medical literature in the 1970s and 1980s indicate that DES was used for treatment of transgender individuals.[92][93][94]
In 1973, in an attempt to restrict off-label use of DES as a postcoital contraceptive (which had become prevalent at many university health services following publication of an influential study in 1971 in JAMA) to emergency situations such as rape, an FDA Drug Bulletin was sent to all U.S. physicians and pharmacists that said the FDA had approved, under restricted conditions, postcoital contraceptive use of DES.[95]
In 1975, the FDA said it had not actually given (and never did give) approval to any manufacturer to market DES as a postcoital contraceptive, but would approve that indication for emergency situations such as rape or incest if a manufacturer provided patient labeling and special packaging as set out in a FDA final rule published in 1975.[96] To discourage off-label use of DES as a postcoital contraceptive, the FDA in 1975 removed DES 25 mg tablets from the market and ordered the labeling of lower doses (5 mg and lower) of DES still approved for other indications changed to state: "This drug product should not be used as a postcoital contraceptive" in block capital letters on the first line of the physician prescribing information package insert and in a prominent and conspicuous location of the container and carton label.[88][97] In the 1980s, off-label use of the Yuzpe regimen of certain regular combined oral contraceptive pills superseded off-label use of DES as a postcoital contraceptive.[98]
In 1978, the FDA removed postpartum lactation suppression to prevent breast engorgement from their approved indications for DES and other estrogens.[99] In the 1990s, the only approved indications for DES were treatment of advanced prostate cancer and treatment of advanced breast cancer in postmenopausal women. The last remaining U.S. manufacturer of DES, Eli Lilly, stopped making and marketing it in 1997.
Lawsuits
In the 1970s, the negative publicity surrounding the discovery of DES's long-term effects resulted in a huge wave of lawsuits in the United States against its manufacturers. These culminated in a landmark 1980 decision of the Supreme Court of California, Sindell v. Abbott Laboratories, in which the court imposed a rebuttable presumption of market share liability upon all DES manufacturers, proportional to their share of the market at the time the drug was consumed by the mother of a particular plaintiff.
A lawsuit was filed in Boston Federal Court by 53 DES daughters who say their breast cancers were the result of DES being prescribed to their mothers while pregnant with them. Their cases survived a Daubert hearing. In 2013, the Fecho sisters who initiated the breast cancer/DES link litigation agreed to an undisclosed settlement amount on the second day of trial. The remaining litigants have received various settlements.[100]
Society and culture
Alan Turing, the ground breaking cryptographer, founder of computing science and programmable computers, who also proposed the actual theoretical model of biological morphogenesis, was forced onto the medication to induce chemical castration as a punitive "treatment" for homosexual behaviour, shortly before he died in ambiguous circumstances.[101]
Veterinary use
Canine incontinence
DES has been very successful in treating female canine incontinence stemming from poor sphincter control. It is still available from compounding pharmacies, and at the low (1 mg) dose, does not have the carcinogenic properties that were so problematic in humans.[102] It is generally administered once a day for seven to ten days and then once every week as needed.
Livestock growth promotion
The greatest usage of DES was in the livestock industry, used to improve feed conversion in beef and poultry. During the 1960s, DES was used as a growth hormone in the beef and poultry industries. It was later found to cause cancer by 1971, but was not phased out until 1979.[103][104] When DES was discovered to be harmful to humans, it was moved to veterinary use.
References
- Bruce Chabner; Dan Louis Longo (1996). Cancer Chemotherapy and Biotherapy: Principles and Practice. Lippincott-Raven Publishers. p. 186. ISBN 978-0-397-51418-2.
Piperazine estrone sulfate and micronized estradiol were equipotent with respect to increases in SHBG, whereas [...] DES was 28.4-fold more potent [...]. With respect to decreased FSH, [...] DES was 3.8-fold, and ethinyl estradiol was 80 to 200-fold more potent than was piperazine estrone sulfate. The dose equivalents for ethinyl estradiol (50 µg) and DES (1 mg) reflect these relative potencies.220 [...] DES, a potent synthetic estrogen (Fig. 6-12), is absorbed well after an oral dosage. Patients given 1 mg of DES daily had plasma concentrations at 20 hours ranging from 0.9 to 1.9 ng per mL. The initial half-life of DES is 80 minutes, with a secondary half-life of 24 hours.222 The principal pathways of metabolism are conversion to the glucuronide and oxidation. The oxidative pathways include aromatic hydroxylation of the ethyl side chains and dehydrogenation to (Z,Z)-dienestrol, producing transient quinone-like intermediates that react with cellular macromolecules and cause genetic damage in eukaryotic cells.223 Metabolic activation of DES may explain its well-established carcinogenic properties.224
- Oelschläger, Herbert; Rothley, Dietrich; Dunzendorfer, Udo (1988). "New Results on the Pharmacokinetics of Fosfestrol". Urologia Internationalis. 43 (1): 15–23. doi:10.1159/000281427. ISSN 1423-0399.
- Droz JP, Kattan J, Bonnay M, Chraibi Y, Bekradda M, Culine S (February 1993). "High-dose continuous-infusion fosfestrol in hormone-resistant prostate cancer". Cancer. 71 (3 Suppl): 1123–30. doi:10.1002/1097-0142(19930201)71:3+<1123::AID-CNCR2820711434>3.0.CO;2-T. PMID 8428334.
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 396–. ISBN 978-1-4757-2085-3.
- Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Elizabeth Siegel Watkins (16 April 2007). The Estrogen Elixir: A History of Hormone Replacement Therapy in America. JHU Press. pp. 26–. ISBN 978-0-8018-8602-7.
- Veurink M, Koster M, Berg LT (June 2005). "The history of DES, lessons to be learned". Pharm World Sci. 27 (3): 139–43. doi:10.1007/s11096-005-3663-z. PMID 16096877.
- "DES Update: For Consumers". United States Department of Health and Human Services: Centers for Disease Control and Prevention. Retrieved 2011-06-30.
- "Diethylstilbestrol (DES) and Cancer". National Cancer Institute. Retrieved 2011-06-30.
- Arnold, Amanda (January 5, 2017). "The Devastating Effects of a 1940s 'Wonder Pill' Haunt Women Generations Later". Broadly.
- Coelingh Bennink HJ (April 2004). "Are all estrogens the same?". Maturitas. 47 (4): 269–275. doi:10.1016/j.maturitas.2003.11.009. PMID 15063479.
- Noller KL, Fish CR (July 1974). "Diethylstilbestrol usage: Its interesting past, important present, and questionable future". Med. Clin. North Am. 58 (4): 793–810. doi:10.1016/S0025-7125(16)32122-8. PMID 4276416.
- Helmuth Vorherr (2 December 2012). The Breast: Morphology, Physiology, and Lactation. Elsevier Science. pp. 201–203. ISBN 978-0-323-15726-1.
- Thompson IM (2001). "Flare Associated with LHRH-Agonist Therapy". Rev Urol. 3 Suppl 3: S10–4. PMC 1476081. PMID 16986003.
- Scaletscky R, Smith JA (April 1993). "Disease flare with gonadotrophin-releasing hormone (GnRH) analogues. How serious is it?". Drug Saf. 8 (4): 265–70. doi:10.2165/00002018-199308040-00001. PMID 8481213.
- Kreis W, Ahmann FR, Jordan VC, de Haan H, Scott M (October 1988). "Oestrogen pre-treatment abolishes luteinising hormone-releasing hormone testosterone stimulation". Br J Urol. 62 (4): 352–4. doi:10.1111/j.1464-410X.1988.tb04364.x. PMID 2973364.
- Stein BS, Smith JA (April 1985). "DES lead-in to use of luteinizing hormone releasing hormone analogs in treatment of metastatic carcinoma of prostate". Urology. 25 (4): 350–3. doi:10.1016/0090-4295(85)90484-4. PMID 3920802.
- Fernandez del Moral P, Litjens TT, Weil EH, Debruyne FM (August 1988). "Can combined DES and LHRH depot therapy (ICI 118630) prevent endocrinologic and clinical flare-up in metastatic prostate cancer?". Urology. 32 (2): 137–40. doi:10.1016/0090-4295(88)90316-0. PMID 2969641.
- Bruchovsky N, Goldenberg SL, Akakura K, Rennie PS (September 1993). "Luteinizing hormone-releasing hormone agonists in prostate cancer. Elimination of flare reaction by pretreatment with cyproterone acetate and low-dose diethylstilbestrol". Cancer. 72 (5): 1685–91. doi:10.1002/1097-0142(19930901)72:5<1685::AID-CNCR2820720532>3.0.CO;2-3. PMID 7688656.
- Kotake T, Usami M, Akaza H, Koiso K, Homma Y, Kawabe K, Aso Y, Orikasa S, Shimazaki J, Isaka S, Yoshida O, Hirao Y, Okajima E, Naito S, Kumazawa J, Kanetake H, Saito Y, Ohi Y, Ohashi Y (November 1999). "Goserelin acetate with or without antiandrogen or estrogen in the treatment of patients with advanced prostate cancer: a multicenter, randomized, controlled trial in Japan. Zoladex Study Group". Jpn. J. Clin. Oncol. 29 (11): 562–70. doi:10.1093/jjco/29.11.562. PMID 10678560.
- H.J. Buchsbaum (6 December 2012). The Menopause. Springer Science & Business Media. pp. 60–. ISBN 978-1-4612-5525-3.
- Reis LO, Zani EL, García-Perdomo HA (June 2018). "Estrogen therapy in patients with prostate cancer: a contemporary systematic review". Int Urol Nephrol. 50 (6): 993–1003. doi:10.1007/s11255-018-1854-5. PMID 29600433.
- Turo R, Smolski M, Esler R, Kujawa ML, Bromage SJ, Oakley N, Adeyoju A, Brown SC, Brough R, Sinclair A, Collins GN (February 2014). "Diethylstilboestrol for the treatment of prostate cancer: past, present and future". Scand J Urol. 48 (1): 4–14. doi:10.3109/21681805.2013.861508. PMID 24256023.
- Bosset PO, Albiges L, Seisen T, de la Motte Rouge T, Phé V, Bitker MO, Rouprêt M (December 2012). "Current role of diethylstilbestrol in the management of advanced prostate cancer". BJU Int. 110 (11 Pt C): E826–9. doi:10.1111/j.1464-410X.2012.11206.x. PMID 22578092.
- Scherr DS, Pitts WR (November 2003). "The nonsteroidal effects of diethylstilbestrol: the rationale for androgen deprivation therapy without estrogen deprivation in the treatment of prostate cancer". J. Urol. 170 (5): 1703–8. doi:10.1097/01.ju.0000077558.48257.3d. PMID 14532759.
- Oh WK (September 2002). "The evolving role of estrogen therapy in prostate cancer". Clin Prostate Cancer. 1 (2): 81–9. doi:10.3816/cgc.2002.n.009. PMID 15046698.
- Malkowicz SB (August 2001). "The role of diethylstilbestrol in the treatment of prostate cancer". Urology. 58 (2 Suppl 1): 108–13. PMID 11502463.
- Cox RL, Crawford ED (December 1995). "Estrogens in the treatment of prostate cancer". J. Urol. 154 (6): 1991–8. doi:10.1016/s0022-5347(01)66670-9. PMID 7500443.
- Lycette JL, Bland LB, Garzotto M, Beer TM (December 2006). "Parenteral estrogens for prostate cancer: can a new route of administration overcome old toxicities?". Clin Genitourin Cancer. 5 (3): 198–205. doi:10.3816/CGC.2006.n.037. PMID 17239273.
- Coelingh Bennink HJ, Verhoeven C, Dutman AE, Thijssen J (January 2017). "The use of high-dose estrogens for the treatment of breast cancer". Maturitas. 95: 11–23. doi:10.1016/j.maturitas.2016.10.010. PMID 27889048.
- Marselos M, Tomatis L (1992). "Diethylstilboestrol: I, Pharmacology, Toxicology and carcinogenicity in humans". Eur. J. Cancer. 28A (6–7): 1182–9. PMID 1627392.
- Ellis, MJ; Dehdahti, F; Kommareddy, A; Jamalabadi-Majidi, S; Crowder, R; Jeffe, DB; Gao, F; Fleming, G; Silverman, P; Dickler, M; Carey, L; Marcom, PK (2014). "A randomized phase 2 trial of low dose (6 mg daily) versus high dose (30 mg daily) estradiol for patients with estrogen receptor positive aromatase inhibitor resistant advanced breast cancer". Cancer Research. 69 (2 Supplement): 16. doi:10.1158/0008-5472.SABCS-16. ISSN 0008-5472.
- Swyer GI (April 1959). "The oestrogens". Br Med J. 1 (5128): 1029–31. doi:10.1136/bmj.1.5128.1029. PMC 1993181. PMID 13638626.
[Diethylstilbestrol] suffers from the serious drawback that in doses above 1 mg. a day it is likely to produce nausea, vomiting, abdominal discomfort, headache, and bloating in a proportion of patients varyingly estimated from 15 to 50%.
- Phillips I, Shah SI, Duong T, Abel P, Langley RE (2014). "Androgen Deprivation Therapy and the Re-emergence of Parenteral Estrogen in Prostate Cancer". Oncol Hematol Rev. 10 (1): 42–47. doi:10.17925/ohr.2014.10.1.42. PMC 4052190. PMID 24932461.
- Di Lorenzo G, Autorino R, Perdonà S, De Placido S (December 2005). "Management of gynaecomastia in patients with prostate cancer: a systematic review". Lancet Oncol. 6 (12): 972–9. doi:10.1016/S1470-2045(05)70464-2. PMID 16321765.
- Del Castillo, E. B.; Argonz, J. (1954). "Oestrogen treatment in cases of rudimentary ovary syndrome". Acta Endocrinologica. 15 (4): 299–312. doi:10.1530/acta.0.0150299. ISSN 0804-4643.
- A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 720–. ISBN 978-3-642-96158-8.
- Davis, M.Edward; Boynton, Melbourne W. (1941). "Indications, clinical use and toxicity of 4-4' dihydroxy diethyl stilbene". The Journal of Clinical Endocrinology & Metabolism. 1 (4): 339–345. doi:10.1210/jcem-1-4-339. ISSN 0021-972X.
- Davis, M. Edward; Boynton, M. W.; Ferguson, J. H.; Rothman, S. (1945). "Studies on Pigmentation of Endocrine Origin1". The Journal of Clinical Endocrinology & Metabolism. 5 (3): 138–146. doi:10.1210/jcem-5-3-138. ISSN 0021-972X.
- Lewis RM (December 1939). "The Clinical Use of Stilbestrol, A Synthetic Estrogen: Preliminary Report". Yale J Biol Med. 12 (2): 235–8. PMC 2602231. PMID 21433876.
- Lisser, H.; Curtis, L. E.; Escamilla, R. F.; Goldberg, Minnie B. (1947). "The syndrome of congenitally aplastic ovaries with sexual infantilism, high urinary gonadotropins, short stature and other congenital abnormalities: tabular presentation of twenty-five previously unpublished cases". The Journal of Clinical Endocrinology & Metabolism. 7 (10): 665–687. doi:10.1210/jcem-7-10-665. ISSN 0021-972X.
- Bamigboye AA, Morris J (2003). "Oestrogen supplementation, mainly diethylstilbestrol, for preventing miscarriages and other adverse pregnancy outcomes". Cochrane Database Syst Rev (3): CD004353. doi:10.1002/14651858.CD004353. PMID 12918007.
- Marselos, M, and Tomatis, L. “Diethylstilboestrol: II, Pharmacology, Toxicology and Carcinogenicity in Experimental Animals.” European Journal of Cancer 29.1 (1993): 149–155.
- Kioumourtzoglou MA, Coull BA, O'Reilly ÉJ, Ascherio A, Weisskopf MG. Association of Exposure to Diethylstilbestrol During Pregnancy With Multigenerational Neurodevelopmental Deficits. JAMA Pediatr. 2018;172(7):670–677. doi:10.1001/jamapediatrics.2018.0727
- Titus-Ernstoff L, Troisi R, Hatch EE, Wise LA, Palmer J, Hyer M, Kaufman R, Adam E, Strohsnitter W, Noller K, Herbst AL, Gibson-Chambers J, Hartge P, Hoover RN Int J Epidemiol. 2006 Aug; 35(4):862-8. DOI: 10.1093/ije/dyl106
- Kalfa N, Paris F, Soyer-Gobillard MO, Daures JP, Sultan C. Fertil Steril. 2011 Jun 30; 95(8):2574-7. DOI: 10.1016/j.fertnstert.2011.02.047
- Carter AC, Sedransk N, Kelley RM, Ansfield FJ, Ravdin RG, Talley RW, Potter NR (May 1977). "Diethylstilbestrol: recommended dosages for different categories of breast cancer patients. Report of the Cooperative Breast Cancer Group". JAMA. 237 (19): 2079–8. doi:10.1001/jama.1977.03270460065023. PMID 576887.
- Jordan VC (2013). Estrogen Action, Selective Estrogen Receptor Modulators, and Women's Health: Progress and Promise. World Scientific. pp. 143–. ISBN 978-1-84816-958-6.
- Seiler JP, Autrup JL, Autrup H (6 December 2012). Diversification in Toxicology — Man and Environment: Proceedings of the 1997 EUROTOX Congress Meeting Held in Århus, Denmark, June 25–28, 1997. Springer Science & Business Media. pp. 23–. ISBN 978-3-642-46856-8.
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA (March 1997). "Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta". Endocrinology. 138 (3): 863–70. doi:10.1210/endo.138.3.4979. PMID 9048584.
- Coss CC, Jones A, Parke DN, Narayanan R, Barrett CM, Kearbey JD, Veverka KA, Miller DD, Morton RA, Steiner MS, Dalton JT (March 2012). "Preclinical characterization of a novel diphenyl benzamide selective ERα agonist for hormone therapy in prostate cancer". Endocrinology. 153 (3): 1070–81. doi:10.1210/en.2011-1608. PMID 22294742.
- Louis J Denis; Keith Griffiths; Amir V Kaisary; Gerald P Murphy (1 March 1999). Textbook of Prostate Cancer: Pathology, Diagnosis and Treatment: Pathology, Diagnosis and Treatment. CRC Press. pp. 294, 297–. ISBN 978-1-85317-422-3.
- Benno Runnebaum; Thomas Rabe (17 April 2013). Gynäkologische Endokrinologie und Fortpflanzungsmedizin: Band 1: Gynäkologische Endokrinologie. Springer-Verlag. pp. 88–. ISBN 978-3-662-07635-4.
- Wallach, Edward E.; Hammond, Charles B.; Maxson, Wayne S. (1982). "Current status of estrogen therapy for the menopause". Fertility and Sterility. 37 (1): 5–25. doi:10.1016/S0015-0282(16)45970-4. ISSN 0015-0282.
- Scott WW, Menon M, Walsh PC (April 1980). "Hormonal Therapy of Prostatic Cancer". Cancer. 45 Suppl 7: 1929–1936. doi:10.1002/cncr.1980.45.s7.1929. PMID 29603164.
- Muhammad A. Salam (2003). Principles & Practice of Urology: A Comprehensive Text. Universal-Publishers. pp. 684–. ISBN 978-1-58112-412-5.
- Lam JS, Leppert JT, Vemulapalli SN, Shvarts O, Belldegrun AS (January 2006). "Secondary hormonal therapy for advanced prostate cancer". J. Urol. 175 (1): 27–34. doi:10.1016/S0022-5347(05)00034-0. PMID 16406864.
- Jorge Martinez-Manautou; Harry W. Rudel (1966). "Antiovulatory Activity of Several Synthetic and Natural Estrogens". In Robert Benjamin Greenblatt (ed.). Ovulation: Stimulation, Suppression, and Detection. Lippincott. pp. 243–253.
- Herr, F.; Revesz, C.; Manson, A. J.; Jewell, J. B. (1970). "Biological Properties of Estrogen Sulfates": 368–408. doi:10.1007/978-3-642-49793-3_8. Cite journal requires
|journal=(help) - Schröder, Fritz H.; Radlmaier, Albert (2009). "Steroidal Antiandrogens": 325–346. doi:10.1007/978-1-59259-152-7_15. Cite journal requires
|journal=(help) - Goldenberg SL, Bruchovsky N, Rennie PS, Coppin CM (December 1988). "The combination of cyproterone acetate and low dose diethylstilbestrol in the treatment of advanced prostatic carcinoma". J. Urol. 140 (6): 1460–5. doi:10.1016/S0022-5347(17)42073-8. PMID 2973529.
- Goldenberg SL, Bruchovsky N, Gleave ME, Sullivan LD (June 1996). "Low-dose cyproterone acetate plus mini-dose diethylstilbestrol--a protocol for reversible medical castration". Urology. 47 (6): 882–4. doi:10.1016/S0090-4295(96)00048-9. PMID 8677581.
- Greschik H, Flaig R, Renaud JP, Moras D (August 2004). "Structural basis for the deactivation of the estrogen-related receptor gamma by diethylstilbestrol or 4-hydroxytamoxifen and determinants of selectivity". The Journal of Biological Chemistry. 279 (32): 33639–46. doi:10.1074/jbc.M402195200. PMID 15161930.
- Pugeat MM, Dunn JF, Nisula BC (July 1981). "Transport of steroid hormones: interaction of 70 drugs with testosterone-binding globulin and corticosteroid-binding globulin in human plasma". J. Clin. Endocrinol. Metab. 53 (1): 69–75. doi:10.1210/jcem-53-1-69. PMID 7195405.
- Chambers P, Günzel P (12 March 2013). Mechanism of Toxic Action on Some Target Organs: Drugs and Other Substances. Springer Science & Business Media. pp. 276–. ISBN 978-3-642-67265-1.
- Couse JF, Korach KS (December 2004). "Estrogen receptor-alpha mediates the detrimental effects of neonatal diethylstilbestrol (DES) exposure in the murine reproductive tract". Toxicology. 205 (1–2): 55–63. doi:10.1016/j.tox.2004.06.046. PMID 15458790.
- Joseph L. Rabinowitz; Ralph M. Myerson (1967). Topics in Medicinal Chemistry. Wiley-Interscience. p. 16. ISBN 978-0-471-70468-3.
- Friedberg V (October 1951). "Die Behandlung der genitalen Hypoplasie mit intrauterinen Cyren-B-Kristallsuspensionen" [Intrauterine Cyren-B Crystal Suspensions in Therapy of Genital Hypoplasia]. Geburtshilfe Frauenheilkd (in German). 11 (10): 923–30. ISSN 0016-5751. PMID 14926876.
- Camille Georges Wermuth; David Aldous; Pierre Raboisson; Didier Rognan (1 July 2015). The Practice of Medicinal Chemistry. Elsevier Science. pp. 244–245. ISBN 978-0-12-417213-5.
- Sneader W (31 October 2005). Drug Discovery: A History. John Wiley & Sons. pp. 196–197. ISBN 978-0-470-01552-0.
- Ravina E (11 January 2011). T he Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. pp. 177–. ISBN 978-3-527-32669-3.
- Vitamins and Hormones. Academic Press. 1945. pp. 233–. ISBN 978-0-08-086600-0.
- Maximov PY, McDaniel RE, Jordan VC (23 July 2013). Tamoxifen: Pioneering Medicine in Breast Cancer. Springer Science & Business Media. pp. 3–. ISBN 978-3-0348-0664-0.
- Dodds EC, Goldberg L, Lawson W, Robinson R (1938). "Estrogenic activity of certain synthetic compounds". Nature. 141 (3562): 247–8. doi:10.1038/141247b0.
- Dodds EC (1957). Biochemical contributions to endocrinology; experiments in hormonal research. Stanford: Stanford University Press. OCLC 1483899.
- Meyers R (1983). D.E.S., the bitter pill. New York: Seaview/Putnam. ISBN 0-399-31008-8.
- Langston N (2010). Toxic bodies: Hormone disruptors and the legacy of DES. New Haven, CT: Yale University Press. ISBN 978-0-300-13607-4.
- Seaman B (2003). The greatest experiment ever performed on women: Exploding the estrogen myth. New York: Hyperion. ISBN 978-0-7868-6853-7.
- Huggins C, Hodges CV (1972). "Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate". CA. 22 (4): 232–40. doi:10.3322/canjclin.22.4.232. PMID 4625049.
- "Prostate cancer yields to a drug". The New York Times. 15 December 1943. p. 29.
- Aurel Lupulescu (24 October 1990). Hormones and Vitamins in Cancer Treatment. CRC Press. pp. 36–. ISBN 978-0-8493-5973-6.
- The Leuprolide Study Group (November 1984). "Leuprolide versus diethylstilbestrol for metastatic prostate cancer". The New England Journal of Medicine. 311 (20): 1281–6. doi:10.1056/NEJM198411153112004. PMID 6436700.
- Dutton DB (1988). Worse than the disease: pitfalls of medical progress. Cambridge: Cambridge University Press. ISBN 0-521-34023-3.
- Physicians' desk reference to pharmaceutical specialties and biologicals (15th ed.). Oradell NJ: Medical Economics. 1961. p. 625. ISBN 0-00-093447-X.
- Dieckmann WJ, Davis ME, Rynkiewicz LM, Pottinger RE (November 1953). "Does the administration of diethylstilbestrol during pregnancy have therapeutic value?". American Journal of Obstetrics and Gynecology. 66 (5): 1062–81. doi:10.1016/S0002-9378(16)38617-3. PMID 13104505.
- Apfel RJ, Fisher SM (1984). To do no harm: DES and the dilemmas of modern medicine. New Haven: Yale University Press. ISBN 0-300-03192-0.
- United States Food and Drug Administration (1971). "Certain estrogens for oral or parenteral use. Drugs for human use; drug efficacy study implementation". Fed Regist. 36 (217): 21537–8.; 36 FR 21537
- FDA (1975). "Certain estrogens for oral use. Notice of withdrawal of approval of new drug applications". Fed Regist. 40 (25): 5384.; 25 FR 5384
- Zuger A (2009-07-27). "At What Height, Happiness? A Medical Tale". The New York Times. NY Times.
- Council on Drugs (1960). "Androgens and estrogens in the treatment of disseminated mammary carcinoma: retrospective study of nine hundred forty-four patients". JAMA. 172 (12): 1271–83. doi:10.1001/jama.1960.03020120049010.
- Ingle JN, Ahmann DL, Green SJ, Edmonson JH, Bisel HF, Kvols LK, Nichols WC, Creagan ET, Hahn RG, Rubin J, Frytak S (January 1981). "Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer". The New England Journal of Medicine. 304 (1): 16–21. doi:10.1056/NEJM198101013040104. PMID 7001242.
- Goodwin WE, Cummings RH (March 1984). "Squamous metaplasia of the verumontanum with obstruction due to hypertrophy: long-term effects of estrogen on the prostate in an aging male-to-female transsexual". The Journal of Urology. 131 (3): 553–4. doi:10.1016/s0022-5347(17)50493-0. PMID 6199525.
- Lehrman KL (February 1976). "Pulmonary embolism in a transsexual man taking diethylstilbestrol". JAMA. 235 (5): 532–3. doi:10.1001/jama.1976.03260310046024. PMID 946104.
- Seyler LE, Canalis E, Spare S, Reichlin S (July 1978). "Abnormal gonadotropin secretory responses to LRH in transsexual women after diethylstilbestrol priming". The Journal of Clinical Endocrinology and Metabolism. 47 (1): 176–83. doi:10.1210/jcem-47-1-176. PMID 122396.
- Kuchera LK (October 1971). "Postcoital contraception with diethylstilbestrol". JAMA. 218 (4): 562–3. doi:10.1001/jama.218.4.562. PMID 5171004.
- FDA (1975). "Diethylstilbestrol as posticoital oral contraceptive; patient labeling". Fed Regist. 40 (25): 5451–5.; 40 FR 5451
- FDA (1975). "Estrogens for oral or parenteral use. Drugs for human use; drug efficacy study; amended notice". Fed Regist. 40 (39): 8242.; 39 FR 8242
- Hatcher RA, Stewart GK, Stewart F, Guest F, Josephs N, Dale J (1982). Contraceptive Technology 1982–1983. New York: Irvington Publishers. pp. 152–7. ISBN 0-8290-0705-9.
- FDA (1978). "Estrogens for postpartum breast engorgement". Fed Regist. 43 (206): 49564–7.; 43 FR 49564
- Lavoie D (9 January 2013). "DES Pregnancy Drug Lawsuit: Settlement Reached Between Melnick Sisters And Eli Lilly And Co". Huffington Post. Archived from the original on 10 January 2013. Retrieved 19 March 2014.
- West-Taylor Z. "The Alan Turing Law – a Formal Pardon for Unpardonable Homophobia". Affinity magazine. Retrieved 3 December 2016.
- "Urinary Incontinence". Merck Veterinary Manual. Merck Veterinary Manual. Retrieved 30 November 2012.
- Harris RM, Waring RH (June 2012). "Diethylstilboestrol--a long-term legacy". Maturitas. 72 (2): 108–12. doi:10.1016/j.maturitas.2012.03.002. PMID 22464649.
- Gandhi R, Snedeker S (2000-06-01). "Consumer Concerns About Hormones in Food" (PDF). Fact Sheet #37, June 2000. Program on Breast Cancer and Environmental Risk Factors, Cornell University. Archived from the original on 2011-07-19. Retrieved 2011-07-20.
Further reading
- Johnston, Emily (2017). "Poisoned subjects: life writing of DES daughters". Frontiers: A Journal of Women Studies. University of Nebraska Press. 38 (1): 31–63. JSTOR 10.5250/fronjwomestud.38.1.0031.
External links
- Diethylstilbestrol (DES) and Cancer National Cancer Institute
- DES Update from the U.S. Centers for Disease Control and Prevention
- DES Action USA national consumer organization providing comprehensive information for DES-exposed individuals
- DES Booklets from the U.S. National Institutes of Health (circa 1980)
- DES Follow-up Study National Cancer Institute's longterm study of DES-exposed persons (including the DES-AD Project)
- University of Chicago DES Registry of patients with CCA (clear cell adenocarcinoma) of the vagina and/or cervix
- DES Diethylstilbestrol Provides resources and social media links for general DES awareness