Oleuropein
Oleuropein is a glycosylated seco-iridoid, a type of phenolic bitter compound found in green olive skin, flesh and seeds, leaves, and argan oil.[1][2] The term oleuropein is derived from the botanical name of the olive tree, Olea europaea.
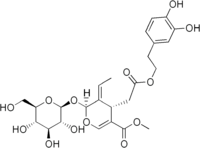 | |
| Names | |
|---|---|
| IUPAC name
(4S,5E,6S)-4-{2-[2-(3,4-dihydroxyphenyl)ethoxy]-2-oxoethyl}-5-ethylidene-6-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)-2-tetrahydropyranyl]oxy}-4H-pyran-3-carboxylic acid methyl ester | |
| Other names
2-(3,4-Dihydroxyphenyl)ethyl [(2S,3E,4S)-3-ethylidene-2-(β-D-glucopyranosyloxy)-5-(methoxycarbonyl)-3,4-dihydro-2H-pyran-4-yl]acetate | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.046.466 |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C25H32O13 |
| Molar mass | 540.51 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Because of its bitter taste, oleuropein must be completely removed or decomposed to make olives edible. During processing of bitter and inedible green olives for consumption as table olives, oleuropein is removed from olives by immersion in lye.[3][4]
Chemical treatment
Oleuropein consists of a molecule of elenolic acid linked to the orthodiphenol hydroxytyrosol by an ester bond, and to a molecule of glucose by a glycosidic bond.[5] Alkaline conditions favor the elimination, or directly the decomposition, of oleuropein from the tissues of fresh green olives immersed in a lye solution. Two mechanisms occur simultaneously: first, at high pH (~ 13.9) in a 3 wt. % NaOH solution, most of the phenolic groups (pKa ≈ 10) present in the oleuropein molecule are deprotonated and present in a dissociated state. The ionized phenolate groups significantly increase the solubility of the molecule in the tissue of the olives. The oleuropein can then more easily diffuse out of the fruits and is released into the lye solution.
Second, under alkaline conditions, the oleuropein molecule is chemically hydrolyzed into hydroxytyrosol and elenolic acid by the breakdown of the ester and glycosidic bonds.[6][7] At high pH, as phenols and polyphenols, the molecule is sensitive to oxidation and can degrade faster, while olives turn black as during their normal ripening, if the solution is oxygenated by air injection (alkaline oxidation of olives also called the California process).[8][9]
The lye solution is replaced several times by a fresh one until the bitter taste has completely disappeared. An alternative process uses an amberlite macroporous resins to trap the oleuropein molecule directly from the solution, giving the advantage to reduce waste water while capturing the extracted molecules.[10][11]
Enzymatic hydrolysis during the maturation of olives is also an important process for the decomposition of oleuropein and elimination of its bitter taste.[7][12]
Green olive blackening
Green olives may be treated industrially with ferrous gluconate (0.4 wt. %)[8] to change their color to black.[13] Gluconate, an edible oxidation product of glucose, is used as non-toxic reactant to maintain Fe2+ in solution. When in contact with polyphenols, the ferrous ions form a black complex, giving the final color of the treated olives.[10][11][8] Black olives treated with iron(II) gluconate are also depleted in hydroxytyrosol, as iron salts are catalysts for its oxidation.[14]
See also
- Elenolic acid (a marker for maturation of olives)
- Hydroxytyrosol
- Oleocanthal
- Olive leaf
- Olive: Traditional fermentation and curing
References
- Z. Charrouf and D. Guillaume (2007). "Phenols and polyphenols from Argania spinosa". American Journal of Food Technology. 2 (7): 679–683. doi:10.3923/ajft.2007.679.683.CS1 maint: uses authors parameter (link)
- Rupp R. (1 July 2016). "The bitter truth about olives". National Geographic. Retrieved 24 June 2019.
- "How olives are made". California Olive Committee. 2017. Retrieved 5 August 2017.
- Colmagro S., Collins G., and Sedgley M. "Processing technology of the table olive" (PDF). Retrieved 25 June 2019.CS1 maint: uses authors parameter (link)
- Panizzi, L.; Scarpati, M.L.; Oriente, E.G. (1960). "Structure of the bitter glucoside oleuropein. Note II". Gazzetta Chimica Italiana. 90: 1449–1485.
- Yuan, Jiao-Jiao; Wang, Cheng-Zhang; Ye, Jian-Zhong; Tao, Ran; Zhang, Yu-Si (2015). "Enzymatic hydrolysis of oleuropein from Olea Europea (olive) leaf extract and antioxidant activities". Molecules. 20 (2): 2903–2921. doi:10.3390/molecules20022903. ISSN 1420-3049. PMC 6272143. PMID 25679050.
- Ramírez, Eva; Brenes, Manuel; García, Pedro; Medina, Eduardo; Romero, Concepción (2016). "Oleuropein hydrolysis in natural green olives: Importance of the endogenous enzymes" (PDF). Food Chemistry. 206: 204–209. doi:10.1016/j.foodchem.2016.03.061. hdl:10261/151764. ISSN 0308-8146. PMID 27041317.
- El-Makhzangy, Attya; Ramadan-Hassanien, Mohamed Fawzy; Sulieman, Abdel-Rahman Mohamed (2008). "Darkening of brined olives by rapid alkaline oxidation". Journal of Food Processing and Preservation. 32 (4): 586–599. doi:10.1111/j.1745-4549.2008.00198.x. ISSN 0145-8892.
- Ziena, H.M.S.; Youssef, M.M.; Aman, M.E. (1997). "Quality attributes of black olives as affected by different darkening methods". Food Chemistry. 60 (4): 501–508. doi:10.1016/S0308-8146(96)00354-8. ISSN 0308-8146.
- "A 'greener' way to take the bitterness out of olives". phys.org. Retrieved 23 June 2019.
- Johnson, Rebecca; Mitchell, Alyson E. (2019). "Use of Amberlite macroporous resins to reduce bitterness in whole olives for improved processing sustainability". Journal of Agricultural and Food Chemistry. 67 (5): 1546–1553. doi:10.1021/acs.jafc.8b06014. ISSN 0021-8561.
- Restuccia, Cristina; Muccilli, Serena; Palmeri, Rosa; Randazzo, Cinzia L.; Caggia, Cinzia; Spagna, Giovanni (2011). "An alkaline ß-glucosidase isolated from an olive brine strain of Wickerhamomyces anomalus". FEMS Yeast Research. 11 (6): 487–493. doi:10.1111/j.1567-1364.2011.00738.x. ISSN 1567-1356.
- Kumral, A.; Basoglu, F. (2008). "Darkening methods used in olive processing". Acta Horticulturae (791): 665–668. doi:10.17660/ActaHortic.2008.791.101. ISSN 0567-7572.
- Vincenzo Marsilio; Cristina Campestre; Barbara Lanza (July 2001). "Phenolic compounds change during California-style ripe olive processing". Food Chemistry. 74 (1): 55–60. doi:10.1016/S0308-8146(00)00338-1.