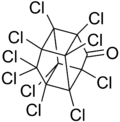
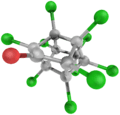
Kepone
Kepone, also known as chlordecone, is an organochlorine compound and a colourless solid. This compound is an obsolete insecticide related to Mirex and DDT. Its use was so disastrous that it is now prohibited in the western world, but only after many millions of kilograms had been produced.[3] Kepone is a known persistent organic pollutant (POP), classified among the "dirty dozen" and banned globally by the Stockholm Convention on Persistent Organic Pollutants as of 2011.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
decachloropentacyclo[5.3.0.02.6.03.9.04.8]decan-5-one[1] | |
| Other names
Chlordecone Clordecone Merex CAS name: 1,1a,3,3a,4,5,5,5a,5b,6-decachlorooctahydro-1,3,4-metheno-2H-cyclobuta[cd]pentalen-2-one | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.093 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C10Cl10O |
| Molar mass | 490.633 g/mol |
| Appearance | tan to white crystalline solid |
| Odor | odorless |
| Density | 1.6 g/cm3 |
| Melting point | 349 °C (660 °F; 622 K) (decomposes) |
Solubility in water |
0.27 g/100 mL |
| Solubility | soluble in acetone, ketone, acetic acid slightly soluble in benzene, hexane |
| log P | 5.41 |
| Vapor pressure | 3.10−7 kPa |
| Thermochemistry | |
Std molar entropy (S |
764 J/K mol |
Std enthalpy of formation (ΔfH⦵298) |
-225.9 kJ/mol |
| Hazards | |
| Main hazards | carcinogen[2] |
| Flash point | Non-flammable[2] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
95 mg/kg (rat, oral) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[2] |
REL (Recommended) |
Ca TWA 0.001 mg/m3[2] |
IDLH (Immediate danger) |
N.D.[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Toxicology
The LC50 (LC = lethal concentration) is 35 μg/ L for Etroplus maculatus,[4] 0.022–0.095 mg/kg for blue gill and trout. Kepone bioaccumulates in animals by factors up to a million-fold. Workers with repeated exposure suffer severe convulsions resulting from degradation of the synaptic junctions.[3] Chronic lower level exposure causes prostate cancer.
Kepone has been found to act as an agonist of the GPER (GPR30).[5]
History
In the US, kepone was produced by Allied Signal Company and LifeSciences Product Company in Hopewell, Virginia. The improper handling and dumping of the substance into the nearby James River (U.S.) in the 1960s and 1970s drew national attention to its toxic effects on humans and wildlife. The product is similar to DDT and is a degradation product of Mirex.[3] The history of Kepone incidents are reviewed in Who's Poisoning America?: Corporate Polluters and Their Victims in the Chemical Age (1982). In 2009, Kepone was included in the Stockholm Convention on persistent organic pollutants, which bans its production and use worldwide.[6]
Case studies
James River estuary
In 1975, Governor Mills Godwin Jr. shut down the James River to fishing for 100 miles, from Richmond to the Chesapeake Bay. This ban remained in effect for 13 years, until efforts to clean up the river began to show results.[7]
Due to the pollution risks, many fishermen, marinas, seafood businesses, and restaurants, along with their employees along the river suffered economic losses. In 1981, a large group of these entities sued Allied Chemical in federal district court (Eastern District of Virginia), claiming special economic damages from Allied's negligent damage to the fish and wildlife.[8] In a case that sometimes appears in law school courses on Remedies, the court rejected the traditional "economic-loss rule", which requires physical impact causing personal injury or property damage in order to receive economic damages, and instead allowed a limited group of the plaintiffs—the fishing boat owners, the marinas, and the bait and tackle shops—to recover economic damages from Allied Chemical.
French Antilles
The French island of Martinique is heavily contaminated with kepone,[9] following years of its unrestricted use on banana plantations.[10][11] Despite a 1990 ban of the substance by France, the economically powerful planter community lobbied intensively to gain the power to continue using kepone until 1993. They had argued that no alternative pesticide was available, which has since been disputed. The nearby island of Guadeloupe is also contaminated, but to a lesser extent. Since 2003, local authorities have restricted cultivation of crops because the soil has been seriously contaminated by kepone. Guadeloupe has one of the highest prostate cancer diagnosis rates in the world.[12]
In popular culture
- Kepone was the name of an American indie rock band from Richmond, Virginia formed in 1991.
- The Dead Kennedys recorded a song named "Kepone Factory", a satire of the controversy surrounding Allied Signal and their negligence regarding employee safety, for their 1981 album In God We Trust, Inc..
Synthesis
Kepone is made by dimerizing hexachlorocyclopentadiene and hydrolyzing to a ketone.[13]
References
- IUPAC Agrochemical information.
- NIOSH Pocket Guide to Chemical Hazards. "#0365". National Institute for Occupational Safety and Health (NIOSH).
- Robert L. Metcalf "Insect Control" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, Wienheim, 2002. doi:10.1002/14356007.a14_263
- Asifa KP, Chitra KC. (2015). Determination of Median Lethal Concentration (LC50) and Behavioral Effects of Chlordecone in the Cichlid fish, Etroplus maculatus. Int J. Sci.Res.4 (3):1473-75
- Prossnitz, Eric R.; Barton, Matthias (2014). "Estrogen biology: New insights into GPER function and clinical opportunities". Molecular and Cellular Endocrinology. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. ISSN 0303-7207. PMC 4040308. PMID 24530924.
- Stockholm Convention. Listing of POPs in the Stockholm Convention: Annex A (Elimination). 2011; Available from: http://chm.pops.int/Convention/The%20POPs/tabid/673/language/fr-CH/Default.aspx.
- Jack Cooksey, "What's in the Water?", Richmond Magazine, June 2007, accessed 13 June 2012.
- Pruitt v. Allied Chemical Corp., 523 F. Supp. 975 (E.D. Va. 1981).
- Durimel A.; et al. (2013). "pH dependence of chlordecone adsorption on activated carbons and role of adsorbent physico-chemical properties". Chemical Engineering Journal. 229: 239–349. doi:10.1016/j.cej.2013.03.036.
- Wong, Alfred; Ribero, Christine (26 March 2014). "Alternative Agricultural Cropping Options for Chlordecone-Polluted Martinique". Études Caribéennes (26). doi:10.4000/etudescaribeennes.6710.

- Agard-Jones, Vanessa (2013-11-01). "Bodies in the System". Small Axe. 17 (3(42)): 182–192. doi:10.1215/07990537-2378991. ISSN 0799-0537.
- "France: Island Paradise With Contaminated Drinking Water". European Journal. Deutsche Welle. 26 May 2010. Archived from the original on 2010-05-27.
- Survey of Industrial Chemistry by Philip J. Chenier (2002), p. 484.
External links
- Terradaily: Pesticide blamed for 'health disaster' in French Caribbean
- EPA releases a Toxicological Review of Kepone (External Review Draft) for public comment - 01/2008
- CDC - NIOSH Pocket Guide to Chemical Hazards