Orestrate
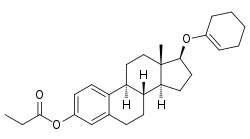
Orestrate (INN), also known as estradiol 3-propionate 17β-(1-cyclohexenyl) ether, is an estrogen medication and estrogen ester which was never marketed.[1][2][3][4] It is the C3 propanoyl, 17β-(1-cyclohexenyl) ether and ester of estradiol.[1][3]
 | |
| Clinical data | |
|---|---|
| Other names | Estradiol 3-propionate 17β-(1-cyclohexenyl) ether; 17β-(Cyclohexen-1-yloxy)-estra-1,3,5(10)-trien-3-ol propionate |
| Drug class | Estrogen; Estrogen ester |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C27H36O3 |
| Molar mass | 408.573 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 898, 905. ISBN 978-1-4757-2085-3.
- P. H. List; L. Hörhammer (12 March 2013). Chemikalien und Drogen Teil A: N-Q. Springer-Verlag. pp. 331–. ISBN 978-3-642-65035-2.
- Edward B. Roche; Academy of Pharmaceutical Sciences. Medicinal Chemistry Section (1977). Design of biopharmaceutical properties through prodrugs and analogs: a symposium. The Academy. p. 7. ISBN 978-0-917330-16-2.
- Galletti, F.; Gardi, R. (1974). "Effect of two orally active estradiol derivatives on sulfobromphthalein retention in rats". Pharmacological Research Communications. 6 (2): 135–145. doi:10.1016/S0031-6989(74)80021-4. ISSN 0031-6989.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.