Polyestriol phosphate
Polyestriol phosphate (PE3P, SEP), sold under the brand names Gynäsan, Klimadurin, and Triodurin, is an estrogen medication which was previously used in menopausal hormone therapy (i.e., to treat menopausal symptoms in postmenopausal women) in Germany but is now no longer available.[1][2][3][4][5][6][7][8][9][10] Its effects on the vagina, uterus, pregnancy, prostate gland, coagulation, and fibrinolysis, as well as on mammary and endometrial cancer risk, have been studied.[11][12][13][14][15][16][17][18][19] PE3P has been used at dosages of 40 to 80 mg per month by intramuscular injection.[8] A single 50 mg injection of PE3P has a duration of approximately one month and a single 80 mg injection has a duration of approximately two months.[3] PE3P is similar to polyestradiol phosphate and is, likewise, an estrogen ester – specifically, an ester and prodrug of estriol – in the form of a polymer with phosphate linkers.[1][2][3][7] When adjusted for differences in molecular weight, it contains the equivalent of about 80% of the amount of estriol.[19] The medication was developed and marketed by the Swedish pharmaceutical company Leo Läkemedel AB.[1][7]
| Estrogen | Form | Major brand name(s) | EPD (14 days) | CIC-D (month) | Duration |
|---|---|---|---|---|---|
| Estradiol | Oil solution | – | 40–60 mg | – | 1–10 mg ≈ 1–2 days |
| Aqueous suspensiona | Mego-E | ? | 3.5 mg | 3.5 mg ≈ >5 days | |
| Microspheres | Juvenum-E, Juvenum | ? | – | 1 mg ≈ 30 days | |
| Estradiol benzoate | Oil solution | Progynon-B | 25–35 mg | – | 5 mg ≈ 3–6 days |
| Aqueous suspension | Agofollin-Depot | 20 mg | – | 10 mg ≈ 16–21 days | |
| Estradiol dipropionate | Oil solution | Agofollin, Di-Ovocyclin, Progynon DP | 25–30 mg | – | 5 mg ≈ 5–8 days |
| Estradiol valerate | Oil solution | Delestrogen, Progynon Depot, Mesigyna | 20–30 mg | 5 mg | 5 mg ≈ 7–8 days; 10 mg ≈ 10–14 days; 40 mg ≈ 14–21 days; 100 mg ≈ 21–28 days |
| Estradiol cypionate | Oil solution | Depo-Estradiol, Depofemin | 20–30 mg | – | 5 mg ≈ 11–14 days |
| Aqueous suspensiona | Cyclofem, Lunelle | ? | 5 mg | 5 mg ≈ 14–24 days | |
| Estradiol benzoate butyratea | Oil solution | Redimen, Soluna, Unijab | ? | 10 mg | 10 mg ≈ 21 days |
| Estradiol enanthatea | Oil solution | Perlutal, Topasel, Yectames | ? | 5–10 mg | 10 mg ≈ 20–30 days |
| Estradiol undecylate | Oil solution | Delestrec, Progynon Depot 100 | ? | – | 10–20 mg ≈ 40–60 days; 25–50 mg ≈ 60–120 days |
| Polyestradiol phosphate | Aqueous solution | Estradurin | 40–60 mg | – | 40 mg ≈ 30 days; 80 mg ≈ 60 days; 160 mg ≈ 120 days |
| Estrone | Oil solution | Kestrin, Theelin | ? | – | 1–2 mg ≈ 2–3 days |
| Aqueous suspension | Estrone Aqueous Suspension | ? | – | ? | |
| Estriol | Oil solution | – | ? | – | 1–2 mg ≈ 1–4 days |
| Polyestriol phosphate | Aqueous solution | Gynäsan, Klimadurin, Triodurin | ? | – | 50 mg ≈ 30 days; 80 mg ≈ 60 days |
| Notes: All by intramuscular injection. All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/day (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate is 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Footnotes: a = Available only in combined injectable contraceptives (i.e., not available alone). Sources: See template. | |||||
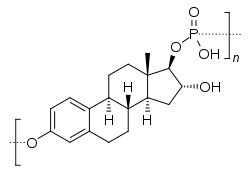 Skeletal structure of polyestriol phosphate | |
| Clinical data | |
|---|---|
| Trade names | Gynäsan, Klimadurin, Triodurin |
| Other names | PE3P; SEP; Poly(estriol phosphate); Estriol phosphate polymer; Estriol polymer with phosphoric acid |
| Routes of administration | Intramuscular injection |
| Drug class | Estrogen; Estrogen ester |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | IM: High |
| Metabolites | Estriol, phosphoric acid, and metabolites of estriol |
| Excretion | Urine (as conjugates) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| Chemical and physical data | |
| Formula | (C18H23O5P)n (n = variable) |
| Molar mass | Polymer: Variable Repeat unit: 350.346 g/mol |
See also
References
- Lauritzen C, Velibese S (September 1961). "Clinical investigations of a long-acting oestriol (polyoestriol phosphate)". Acta Endocrinol. 38 (1): 73–87. doi:10.1530/acta.0.0380073. PMID 13759555.
- Bachmann FF (January 1971). "Behandlung klimakterisher Beschwerden mit Polyöstriolphosphat" [Treatment of menopausal complants with polyoestriol-phosphate. Experiences with Gynäsan injections]. Munch Med Wochenschr (in German). 113 (5): 166–9. PMID 5107471.
- A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 551–. ISBN 978-3-642-96158-8.
The polymer of estradiol or estriol and phosphoric acid has an excellent depot action when given intramuscularly (polyestriol phosphate or polyestradiol phosphate) (Table 16). Phosphoric acid combines with the estrogen molecule at C3 and C17 to form a macromolecule. The compound is stored in the liver and spleen where the estrogen is steadily released by splitting off of the phosphate portion due to the action of alkaline phosphatase. [...] Conjugated estrogens and polyestriol and estradiol phosphate can also be given intravenously in an aqueous solution. Intravenous administration of ovarian hormones offers no advantages, however, and therefore has no practical significance. [...] The following duarations of action have been obtained with a single administration (WlED, 1954; LAURITZEN, 1968): [...] 50 mg polyestradiol phosphate ~ 1 month; 50 mg polyestriol phosphate ~ 1 month; 80 mg polyestriol phosphate ~ 2 months.
- Martin Negwer; Hans-Georg Scharnow (4 October 2001). Organic-chemical drugs and their synonyms: (an international survey). Wiley-VCH. ISBN 978-3-527-30247-5.
8075-01 (6628-01) 37452-43-0 R Polymeric ester with phosphoric acid S Klimadurin, Polyestriol phosphate, Polyostriolphosphat, Triodurin U Depot-estrogen
- William Martindale; Royal Pharmaceutical Society of Great Britain. Dept. of Pharmaceutical Sciences (1993). The Extra Pharmacopoeia. Pharmaceutical Press. p. 2258. ISBN 978-0-85369-300-0.
Polyoestriol Phosphate. [...] ingredient of Klimadurin. [...] Triodurin [...].
- Archiv für Gynäkologie. 1971. p. 206.
Polyoestriol phosphat. Gynäsan (R) pro injectione, 50 mg als Depot.
- Ars Medici. 1971. pp. 194–196, 408, 786.
Klinik in Lund die wirküng von Östriol an einem Urethritismaterial untersucht. Bei dem Präparat, Triodurin1-Leo, das bei der Prüfung verwendet wurde, handelt es sich um ein Polyöstriolphosphat, ein Polyester aus östra-1,3,5(10)-triene-3,16α,17β-triol und Phosphorsäure. Das östriolmolekül wurde mittels Phosphorsäurebrücken in die polymere Form gebracht, und das Molekül ent-hält eine grosse Zahl von Östrioleinheiten (Kön vves, 1965). Das Präparat besitzt [...] Behandlung und Ergebnisse Sämtliche Patientinnen erhielten etwa jeden 2. Monat 50-80 mg Poly-ostriolphosphat. Die Frauen reagierten fast ausnahmslos zufriedenstellend auf diese Behandlung. Die meisten gaben spontane Besserung ihrer übrigen klimak- [...] Eigenschaften Klimadurin F. Ist ein aus natürlichem Oestriol gewonnenes Depot-östrogen. Das wasserlösliche, aber inaktive polymerisierte östriol-phosphat wird durch langsamen Abbau als biologisch wirksame Form kontinuierlich freigesetzt. Die östrogene Wirkung äussert sich fast ausschliesslich in einer Stimullerung der Cervixdrüsen und der Proli- [...]
- Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- W.H. Utian (6 December 2012). The Menopause Manual: A woman’s guide to the menopause. Springer Science & Business Media. pp. 58–. ISBN 978-94-011-7135-9.
- S. Campbell (6 December 2012). The Management of the Menopause & Post-Menopausal Years: The Proceedings of the International Symposium held in London 24–26 November 1975 Arranged by the Institute of Obstetrics and Gynaecology, The University of London. Springer Science & Business Media. pp. 395–. ISBN 978-94-011-6165-7.
In the Federal Republic of Germany between 10 and 20% of all climacteric women are on estrogen treatment. We have the following oral estrogens for a treatment. (t) Conjugated estrogens, (2) estradiol valerate, (3) ethinyl-estradiol and its cyclopentyl-enol ether, (4) stilbestrol, (5) ethinyl-estradiol-methyltestosterone, (6) estriol and estriol succinate, most of them as coated tablets. Several long acting injectable preparations are available: several esters of combined estradiol-testosterone, one of estradiol-dehydroepiandrosterone enanthate and a prolonged polyestriol phosphate are also available. Lastly, depot injections of estradiol- and stilbestrol-esters are on the market.
- Sjöstedt S, Strandh J (1971). "Effect of polyestriol phosphate on the vaginal cytology and uterine endometrium of postmenopausal women". Acta Obstet Gynecol Scand Suppl. 50 (Suppl 9): 30. doi:10.3109/00016347109161433. PMID 5287101.
- Purola E, Vartiainen E (1977). "Effect of long-acting oestriol on the vaginal cytology of postmenopausal women". Ann Chir Gynaecol. 66 (4): 216–8. PMID 907314.
- Fredholm B, Lindskog M (January 1969). "Some effects of a long-acting estriol polymer, polyestriol phosphate". Acta Physiologica Scandinavica: 74.
- Müntzing J (1969). "Effects of polymerized oestrogens on the ventral prostate in rats". Acta Pharmacol Toxicol (Copenh). 27 (6): 417–23. doi:10.1111/j.1600-0773.1969.tb00488.x. PMID 5395730.
- Lutwak-Mann C, Hay MF (1964). "Effect of certain water-soluble oestrogens on rabbit blastocysts". Journal of Endocrinology. 30 (4): ix–x.
- Andersson M, Müntzing J (1971). "Effects of oestrogen on phosphatase activity in the ventral prostate of intact, castrated, and androgen-treated castrated, adult rats". Acta Pharmacol Toxicol (Copenh). 30 (3): 193–202. doi:10.1111/j.1600-0773.1971.tb00650.x. PMID 5171939.
- Gjønnæss, Halvard; Munkeby, Inger; Frølich, Wenche; Vennerød, Anne Marie; Fagerhol, Magne K. (1978). "Effect of Estrogen Treatment on Coagulation and Fibrinolysis in Postmenopausal Women". Gynecologic and Obstetric Investigation. 9 (2–3): 109–123. doi:10.1159/000300974. ISSN 1423-002X.
- Lauritzen, C.; Meier, F. (1984). "Risks of endometrial and mammary cancer morbidity and mortality in long-term oestrogen treatment": 207–216. doi:10.1007/978-94-009-5608-7_20. Cite journal requires
|journal=(help) - Terenius L (February 1971). "Effect of anti-oestrogens on initiation of mammary cancer in the female rat". Eur J Cancer. 7 (1): 65–70. doi:10.1016/0014-2964(71)90096-X. PMID 5576730.