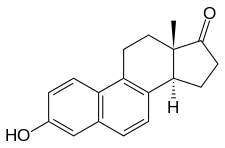
Equilenin
Equilenin, also known as 6,8-didehydroestrone, as well as estra-1,3,5(10),6,8-pentaen-3-ol-17-one, is a naturally occurring steroidal estrogen obtained from the urine of pregnant mares.[1][2] It is used as one of the components in conjugated estrogens (brand name Premarin).[2] It was the first complex natural product to be fully synthesized, in work reported by 1940 by Bachmann and Wilds.[3]
 | |
| Clinical data | |
|---|---|
| Other names | 6,8-Didehydroestrone; Estra-1,3,5(10),6,8-pentaen-3-ol-17-one |
| Routes of administration | By mouth |
| Drug class | Estrogen |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.483 |
| Chemical and physical data | |
| Formula | C18H18O2 |
| Molar mass | 266.339 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Pharmacology
Pharmacodynamics
| Compound | Synonym | Proportion (%) | Relative potency in the vagina (%) | Relative potency in the uterus (%) | RBA for ERα (%) | RBA for ERβ (%) | ERα / ERβ RBA ratio |
|---|---|---|---|---|---|---|---|
| Conjugated estrogens | – | 100 | 38 | 100 | – | – | – |
| Estrone | – | 49.1–61.5 | 30 | 32 | 26 | 52 | 0.50 |
| Equilin | Δ7-Estrone | 22.4–30.5 | 42 | 80 | 13 | 49 | 0.26 |
| 17α-Dihydroequilin | Δ7-17α-Estradiol | 13.5–19.5 | 0.06 | 2.6 | 41 | 32 | 1.30 |
| 17α-Estradiol | – | 2.5–9.5 | 0.11 | 3.5 | 19 | 42 | 0.45 |
| Δ8-Estrone | – | 3.5–3.9 | ? | ? | 19 | 32 | 0.60 |
| Equilenin | Δ6,8-Estrone | 2.2–2.8 | 1.3 | 11.4 | 15 | 20–29 | 0.50–0.75 |
| 17β-Dihydroequilin | Δ7-17β-Estradiol | 0.5–4.0 | 83 | 200 | 113 | 108 | 1.05 |
| 17α-Dihydroequilenin | Δ6,8-17α-Estradiol | 1.2–1.6 | 0.018 | 1.3 | 20 | 49 | 0.40 |
| 17β-Estradiol | – | 0.56–0.9 | 100 | ? | 100 | 100 | 1.00 |
| 17β-Dihydroequilenin | Δ6,8-17β-Estradiol | 0.5–0.7 | 0.21 | 9.4 | 68 | 90 | 0.75 |
| Δ8-17β-Estradiol | – | Small amounts | ? | ? | 68 | 72 | 0.94 |
| Notes: All listed compounds are present in conjugated estrogen products specifically in the form of the sodium salts of the sulfate esters (i.e., as sodium estrone sulfate, sodium equilin sulfate, etc.). Sources: See template. | |||||||
Chemistry
Synthesis
Total synthesis
The synthesis developed by the Bachmann group started from Butenand's ketone[4] – the 7-methoxy structural analog of 1,2,3,4-tetrahydrophenanthren-1-one[5] – and which can be readily prepared from 1,6-Cleve's acid.[6] The approach was based on well-established transformations like the Claisen condensation, the Reformatsky reaction, the Arndt–Eistert reaction, and the Dieckmann condensation.[3] Nicolaou described this preparation as ending the era preceding the post-World War II work of Robert Burns Woodward that introduced enantioselective synthesis;[4] in this synthesis, a mixture of stereoisomers were prepared and then resolved,[6] and the choice of target was partly because of the existence of only two chiral carbons and hence only four stereoisomers.[5]
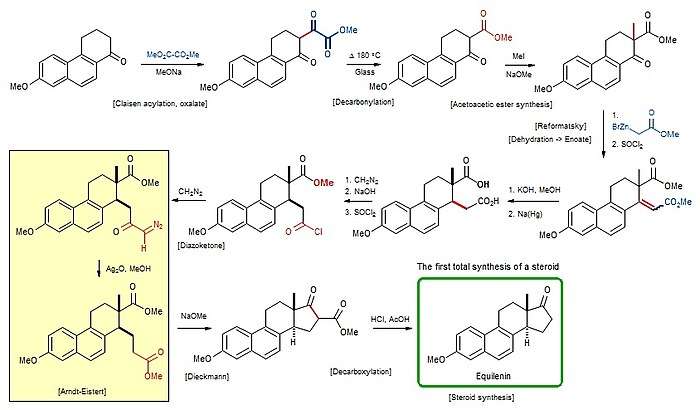
The overall yield of the synthesis was 2.7% based on a twenty-step process starting from Cleve's acid.[6]
See also
References
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 494–. ISBN 978-1-4757-2085-3.
- Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 751–. ISBN 978-1-4511-4847-3.
- Bachmann, Werner E.; Cole, Wayne; Wilds, Alfred L. (1940). "The Total Synthesis of the Sex Hormone Equilenin and Its Stereoisomers". J. Am. Chem. Soc. 62 (4): 824–839. doi:10.1021/ja01861a036.
- Nicolaou, Kyriacos C.; Vourloumis, Dionisios; Winssinger, Nicolas; Baran, Phil S. (2000). "The Art and Science of Total Synthesis at the Dawn of the Twenty-First Century" (PDF). Angew. Chem. Int. Ed. 39 (1): 44–122. doi:10.1002/(SICI)1521-3773(20000103)39:1<44::AID-ANIE44>3.0.CO;2-L. Archived from the original (PDF) on 2017-05-17. Retrieved 2017-07-22.
- Bachmann, Werner E.; Cole, Wayne; Wilds, Alfred L. (1939). "The Total Synthesis of the Sex Hormone Equilenin". J. Am. Chem. Soc. 61 (4): 974–975. doi:10.1021/ja01873a513.
- Nakanishi, Koji (1974). "Steroids". In Nakanishi, Koji; Goto, Toshio; Itô, Shô; Natori, Shinsaku; Nozoe, Shigeo (eds.). Natural Products Chemistry. 1. Academic Press. pp. 421–545.