Fispemifene
Fispemifene (INN, USAN) (developmental code name HM-101) is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was developed for the treatment of male hypogonadism but was abandoned and never marketed.[1][2][3] It reached phase II clinical trials for this indication before development was terminated in March 2016.[1] The drug failed to achieve statistical significance on key effectiveness endpoints in clinical trials and was discontinued by its developer for strategic reasons.[1]
 | |
| Clinical data | |
|---|---|
| Other names | HM-101 |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
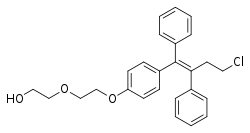
| Formula | C26H27ClO3 |
| Molar mass | 422.9493 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
References
- https://adisinsight.springer.com/drugs/800013715%5B%5D
- Antonio Cano; Joacquim Calaf i Alsina; Jose Luis Duenas-Diez (22 September 2006). Selective Estrogen Receptor Modulators: A New Brand of Multitarget Drugs. Springer Science & Business Media. pp. 52–. ISBN 978-3-540-34742-2.
- Eckhard Ottow; Hilmar Weinmann (8 September 2008). Nuclear Receptors as Drug Targets. John Wiley & Sons. pp. 90–. ISBN 978-3-527-62330-3.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.