Hippulin
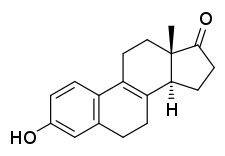
Hippulin, also known as Δ8-14-isoestrone, as well as 14-isoestra-1,3,5(10),8-tetraen-3-ol-17-one, is a naturally occurring estrogen found in horses and an isomer of equilin.[1][2][3][4] The compound, likely in sodium sulfate form, is a component of conjugated estrogens (Premarin), a pharmaceutical extract of the urine of pregnant mares,[1][2][3] though it is present only in small amounts in pregnant mare urine.[5] It has been reported by possess either equivalent estrogenic activity to that of equilin or only slight estrogenic activity.[3] The compound was first described in 1932.[4][3]
 | |
| Clinical data | |
|---|---|
| Other names | Δ8-14-Isoestrone; 8-Dehydro-14-isoestrone; 14-Isoestra-1,3,5(10),8-tetraen-3-ol-17-one |
| Routes of administration | By mouth |
| Drug class | Estrogen |
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C18H20O2 |
| Molar mass | 268.356 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
References
- C. W. Emmens (22 October 2013). Hormone Assay. Elsevier Science. pp. 391–. ISBN 978-1-4832-7286-3.
- H.J. Buchsbaum (6 December 2012). The Menopause. Springer Science & Business Media. pp. 56–. ISBN 978-1-4612-5525-3.
- Banes D, Carol J, Haenni EO (1950). "The resolution of isoequilin A and the identification of compound 3" (PDF). J. Biol. Chem. 187 (2): 557–70. PMID 14803438.
- Girard, H., Sandulesco, G., Fridenson, A., Gaudefroy, C., & Rutgers, J. J. (1932). Sur les Hormones Sexuelles Cristallisées Retirées de l'Urine des Juments Gravides. Compt. Rend. Acad. Sci, 194, 1020.
- Wintersteiner, O. (1937). "Estrogenic Diols from the Urine of Pregnant Mares". Cold Spring Harbor Symposia on Quantitative Biology. 5 (0): 25–33. doi:10.1101/SQB.1937.005.01.003. ISSN 0091-7451.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.