Vaginal epithelium
The vaginal epithelium is the inner lining of the vagina consisting of multiple layers of (squamous) cells.[1][2][3] The basal membrane provides the support for the first layer of the epithelium-the basal layer. The intermediate layers lie upon the basal layer and the superficial layer is the outermost layer of the epithelium.[4][5] Anatomists have described the epithelium as consisting of as many as 40 distinct layers.[6] The mucous found on the epithelium is secreted by the cervix and uterus.[7] The rugae of the epithelium create an involuted surface and result in a large surface area that covers 360 cm3.[8] This large surface area allows the trans-epithelial absorption of some medications via the vaginal route.
| Vaginal epithelium | |
|---|---|
_(4881813679).jpg) The epithelium of the vagina, visible at top, consists of multiple layers of flat cells. | |
| Details | |
| Part of | Vagina |
| Anatomical terminology | |
| This article is one of a series on |
| Epithelia |
|---|
| Squamous epithelial cell |
| Columnar epithelial cell |
| Cuboidal epithelial cell |
| Specialised epithelia |
|
| Other |
In the course of the reproductive cycle, the vaginal epithelium is subject to normal, cyclic changes, that are influenced by estrogen: with increasing circulating levels of the hormone, there is proliferation of epithelial cells along with an increase in the number of cell layers.[9][10] As cells proliferate and mature, they undergo partial cornification.[8][11] Although hormone induced changes occur in the other tissues and organs of the female reproductive system, the vaginal epithelium is more sensitive and its structure is an indicator of estrogen levels.[10][11][12] Some Langerhans cells and melanocytes are also present in the epithelium.[11] The epithelium of the ectocervix is contiguous with that of the vagina, possessing the same properties and function.[13] The vaginal epithelium is divided into layers of cells, including the basal cells, the parabasal cells, the superficial squamous flat cells, and the intermediate cells.[14][15][7] The superficial cells exfoliate continuously and basal cells replace the superficial cells that die and slough off from the stratum corneum.[16][17][18] Under the stratus corneum is the stratum granulosum and stratum spinosum.[19] The cells of the vaginal epithelium retain a usually high level of glycogen compared to other epithelial tissue in the body.[20] The surface patterns on the cells themselves are circular and arranged in longitudinal rows.[6] The epithelial cells of the uterus possess some of the same characteristics of the vaginal epithelium.[21]
Structure
Vaginal epithelium forms transverse ridges or rugae that are most prominent in the lower third of the vagina. This structure of the epithelium results in an increased surface area that allows for stretching.[22][23][8] This layer of epithelium is protective and its uppermost surface of cornified (dead) cells that are unique in that they are permeable to microorganisms that are part of the vaginal flora. The lamina propria of connective tissue is under the epithelium.[4][5]
Cells
| cell type | Features | Diameter | Nuclei | Notes |
|---|---|---|---|---|
| basal cell | round to cylindrical, narrow basophilic cytoplasmic space | 12-14 μm | distinct, 8–10 μm in size | only in case of severe epithelial atrophy and in repair processes after inflammation |
| stratum granulosum | part of the parabasal layer, round to longitudinal oval, cytoplasm basophilic | 20 μm | clear cell nucleus | Frequent glycogen storage, thickened cell margins and decentralized cell nucleus; Predominant cell type in menopausal women[11][23][15][19] |
| stratum spinosum | part of the parabasal layer | [19][15][23] | ||
| intermediate cell | oval to polygonal, cytoplasm basophilic | 30–50 μm | approx. 8 μm, decreasing core-plasma relation with increase in size | in pregnancy : barge-like with thickened cell margin ("navicular cells") |
| superficial squamous flat cells | polygonal, baso- or eosinophilic, transparent, partially keratohyaline granule | 50–60 microns | vesicular and slightly stainable or shrunken | [23][15] |
| stratum corneum | exfoliate, slough off | become detached from the epithelium | [17][18][16] |
Basal cells
The basal layer of the epithelium is the most mitotically active and reproduces new cells.[17] This layer is composed of one layer of cuboidal cells laying on top of the basal membrane.[6]
Parabasal cells
The parabasal cells include the stratum granulousum and the stratum spinosum.[19] In these two layers, cells from the lower basal layer transition from active metabolic activity to death (apoptosis). In these mid-layers of the epithelia, the cells begin to lose their mitochondria and other cell organelles.[17][24] The multiple layers of parabasal cells are polyhedral in shape with prominent nuclei.[6]
Intermediate cells
Intermediate cells make abundant glycogen and store it.[25][26] Estrogen induces the intermediate and superficial cells to fill with glycogen.[18][27] The intermediate cells contain nuclei and are larger than the parabasal cells and more flattened. Some have identified a transitional layer of cells above intermediate layer[6]
Superficial cells
Estrogen induces the intermediate and superficial cells to fill with glycogen.[18][27] Several layers of superficial cells exist that consist large, flattened cells with indistinct nuclei. The superficial cells are exfoliated continuously.[6]
Cell junctions
The junctions between epithelial cells regulate the passage of molecules, bacteria and viruses by functioning as a physical barrier.[13][8] The three types of structural adhesions between epithelial cells are: tight junctions, adherens junctions, and desmosomes. "Tight junctions (zonula occludens) are composed of transmembrane proteins that make contact across the intercellular space and create a seal to restrict transmembrane proteins difusion.[16] of molecules across the epithelial sheet. Tight junctions also have an organizing role in epithelial polarization by limiting the mobility of membrane-bound molecules between the apical and basolateral domains of the plasma membrane of each epithelial cell. Adherens junctions (zonula adherens) connect bundles of actin filaments from cell to cell to form a continuous adhesion belt, usually just below the microfilaments."[13] Junction integrity changes as the cells move to the upper layers of the epidermis.[8]
Mucous
The vagina itself does not contain mucous glands.[28][29] Though mucous is not produced by the vaginal epithelium, mucous originates from the cervix.[7] The cervical mucous that is located inside the vagina can be used to assess fertility in ovulating women.[28] The Bartholin's glands and Skene's glands located at the entrance of the vagina do produce mucous.[30]
Development
The epithelium of the vagina originates from three different precursors during embryonic and fetal development. These are the vaginal squamous epithelium of the lower vagina, the columnar epithelium of the endocervix, and the squamous epithelium of the upper vagina. The distinct origins of vaginal epithelium may impact the understanding of vaginal anomalies.[31] Vaginal adenosis is a vaginal anomaly traced to displacement of normal vaginal tissue by other reproductive tissue within the muscular layer and epithelium of the vaginal wall. This displaced tissue often contains glandular tissue and appears as a raised, red surface.[26]
Cyclic variations
During the luteal and follicular phases of the estrous cycle the structure of the vaginal epithelium varies. The number of cell layers vary during the days of the estrous cycle:
Day 10, 22 layers
Days 12-14, 46 layers
Day 19, 32 layers
Day 24, 24 layers
The glycogen levels in the cells is at its highest immediately before ovulation.[6]
Lytic cells
Without estrogen, the vaginal epithelium is only a few layers thick. Only small round cells are seen that originate directly from the basal layer (basal cells) or the cell layers (parabasal cells) above it. The parabasal cells, which are slightly larger than the basal cells, form a five- to ten-layer cell layer. The parabasal cells can also differentiate into histiocytes or glandular cells. Estrogen also influences the changing ratios of nuclear constituents to cytoplasm. As a result of cell aging, cells with shrunken, seemingly foamy cell nuclei (intermediary cells ) develop from the parabasal cells. These can be categorized by means of the nuclear-plasma relation into "upper" and "deep" intermediate cells.[10] Intermediate cells make abundant glycogen and store it. The further nuclear shrinkage and formation of mucopolysaccharides are distinct characteristics of superficial cells. The mucopolysaccharides form a keratin-like cell scaffold. Fully keratinized cells without a nucleus are called 'floes'.[32][25] Intermediate and superficial cells are constantly exfoliated from the epithelium. The glycogen from these cells is converted to sugars and then fermented by the bacteria of the vaginal flora to lactic acid.[32][27] The cells progress through the cell cycle and then decompose (cytolysis) within a week's time. Cytolysis occurs only in the presence of glycogen-containing cells, that is, when the epithelium is degraded to the upper intermediate cells and superficial cells. In this way, the cytoplasm is dissolved, while the cell nuclei remain.[32]
Epithelial microbiota

Low pH is necessary to control vaginal microbiota. Vaginal epithelial cells have a relatively high concentration of glycogen compared to other epithelial cells of the human body. The metabolism of this complex sugar by the lactobacillus dominated microbiome is responsible for vaginal acidity.[33][34][35]
Function
The cellular junctions of the vaginal epithelium help prevent pathogenic microorganisms from entering the body though some are still able to penetrate this barrier. Cells of the cervix and vaginal epithelium generate a mucous barrier (glycocalyx) in which immune cells reside. In addition, white blood cells provide additional immunity and are able to infiltrate and move through the vaginal epithelium.[13] The epithelium is permeable to antibodies, other immune system cells, and macromolecules. The permeability of epithelium thus provides access for these immune system components to prevent the passage of invading pathogens into deeper vaginal tissue.[8] The epithelium further provides a barrier to microbes the synthesis of antimicrobial peptides (beta-defensins and cathelicidins) and immunoglobulins.[13] Terminally differentiated, superficial keratinocytes extrude the contents of lamellar bodies out of the cell to form a specialized, intercellular lipid envelope that encases the cells of the epidermis and provides a physical barrier to microorganisms.[8]
Clinical significance
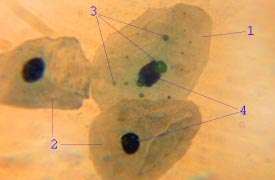
Disease transmission
Sexually transmitted infections, including HIV are rarely transmitted across intact and healthy epithelium. These protective mechanisms are due to: frequent exfoliation of the superficial cells, low pH, and innate and acquired immunity in the tissue. Research into the protective nature of the vaginal epithelium has been recommended as it would help in the design of topical medication and microbicides.[8]
Cancer
There are very rare malignant growths that can originate in the vaginal epithelium.[36] Some are only known through case studies. They are more common in older women.[37]
- Vaginal squamous-cell carcinoma arises from the squamous cells of the epithelium[36]
- Vaginal adenocarcinoma arises from secretory cells in the epithelium[36]
- Clear cell adenocarcinoma of the vagina arises in response to prenatal exposure to diethylstilbestrol[38][39]
- Vaginal melanoma arises from melanocytes in the epithelium[40]
Inflammation
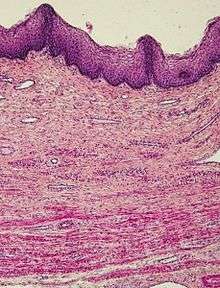
- Candida vaginitis is a fungal infection; the discharge is irritating to the vagina and the surrounding skin.[41]
- Bacterial vaginosis Gardnerella usually causes a discharge, itching and irritation[42][43]
- Aerobic vaginitis thinned reddish vaginal epitherlium, sometimes with erosions or ulcerations and abundant yellowish discharge[44]
Atrophy
The vaginal epithelium changes significantly when estrogen levels decrease at menopause.[45] Atrophic vaginitis[46] usually causes scant odorless discharge with no odor[47]
History
The vaginal epithelium has been studied since 1910 by a number of histologists.[31]
Research
The used of nanoprticles that can penetrate the cervical mucous (present in the vagina) and vaginal epithelium has been investigated to determine if medication can be administered in this manner to provide protection from infection of the Herpes simplex virus.[48] Nanoparticle drug administration into and through the vaginal epithelium to treat HIV infection is also being investigated.[49]
See also
References
- Up to 26 layers have been seen - see Pathology, American Society for Colposcopy and Cervical; Mayeaux, E. J.; Cox, J. Thomas (2011-12-28). Modern Colposcopy Textbook and Atlas. Lippincott Williams & Wilkins. ISBN 9781451153835.
- E R, Weissenbacher (2015-06-02). Immunology of the female genital tract. Heidelberg. p. 16. ISBN 9783642149054. OCLC 868922790.
- Hafez ES, Kenemans P (2012-12-06). Atlas of Human Reproduction: By Scanning Electron Microscopy. Springer Science & Business Media. ISBN 9789401181402.
- Brown L (2012). Pathology of the Vulva and Vagina. Springer Science+Business Media. pp. 6–7. ISBN 978-0857297570. Retrieved February 21, 2014.
- Arulkumaran S, Regan L, Papageorghiou A, Monga A, Farquharson D (2011). Oxford Desk Reference: Obstetrics and Gynaecology. Oxford University Press. p. 471. ISBN 978-0191620874. Retrieved February 21, 2014.
- Hafez ES, Kenemans P (2012-12-06). Atlas of Human Reproduction: By Scanning Electron Microscopy. Springer Science & Business Media. pp. 1–6. ISBN 9789401181402.
- USMLE Step 1 Lecture Notes 2017: Anatomy. Simon and Schuster. 2017. p. 185. ISBN 9781506209463.
- Anderson DJ, Marathe J, Pudney J (June 2014). "The structure of the human vaginal stratum corneum and its role in immune defense". American Journal of Reproductive Immunology. 71 (6): 618–23. doi:10.1111/aji.12230. PMC 4024347. PMID 24661416.
- Nauth HF (2014). Gynäkologische Zytodiagnostik (in German) (2nd ed.). Stuttgart: Georg Thieme. p. 22. ISBN 978-3-13-131092-7.
- Karl Knörr, Henriette Knörr-Gärtner, Fritz K. Beller, Christian Lauritzen (2013), Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion (in German) (3rd ed.), Berlin: Springer, pp. 24–25, ISBN 978-3-642-95584-6CS1 maint: multiple names: authors list (link)
- Pathology AS, Mayeaux EJ, Cox JT (2011-12-28). Modern Colposcopy Textbook and Atlas. Lippincott Williams & Wilkins. ISBN 9781451153835.
- "Vaginal Cytology: Introduction and Index". www.vivo.colostate.edu. Retrieved 2018-02-06.
- Blaskewicz CD, Pudney J, Anderson DJ (July 2011). "Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia". Biology of Reproduction. 85 (1): 97–104. doi:10.1095/biolreprod.110.090423. PMC 3123383. PMID 21471299.
- Dutta DC, Konar H (2014-04-30). DC Dutta's Textbook of Gynecology. JP Medical Ltd. ISBN 9789351520689.
- Mayeaux EJ, Cox TJ (2011). Modern Colposcopy Textbook and Atlas. Lippincott Williams & Wilkins. ISBN 978-1451153835. Retrieved December 11, 2017.
- Beckmann CR (2010). Obstetrics and Gynecology. Lippincott Williams & Wilkins. pp. 241–245. ISBN 978-0781788076.
- Kurman RJ, ed. (2002). Blaustein's Pathology of the Female Genital Tract (5th ed.). Springer. p. 154. ISBN 9780387952031.
- Stanley J. Robboy (2009). Robboy's Pathology of the Female Reproductive Tract. Elsevier Health Sciences. p. 111. ISBN 978-0443074776. Retrieved November 5, 2014.
- Haschek WM, Rousseaux CG, Wallig MA (2009-11-23). Fundamentals of Toxicologic Pathology. Academic Press. ISBN 9780080919324.
- Kurman RJ, ed. (2002). Blaustein's Pathology of the Female Genital Tract (5th ed.). Springer. p. 154. ISBN 9780387952031.
- Yarbrough, Victoria L.; Winkle, Sean; Herbst-Kralovetz, Melissa M. (2015-05-01). "Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications (review)". Human Reproduction Update. 21 (3): 353–377. doi:10.1093/humupd/dmu065. ISSN 1355-4786. PMID 25547201.
- Snell RS (2004). Clinical Anatomy: An Illustrated Review with Questions and Explanations. Lippincott Williams & Wilkins. p. 98. ISBN 978-0-7817-4316-7.
- Dutta DC (2014). DC Dutta's Textbook of Gynecology. JP Medical Ltd. pp. 2–7. ISBN 978-9351520689.
- Gupta R (2011). Reproductive and developmental toxicology. London: Academic Press. p. 1005. ISBN 978-0-12-382032-7.
- Wehrend A (2010). Leitsymptome Gynäkologie und Geburtshilfe beim Hund (in German). Stuttgart: Enke. p. 17. ISBN 978-3-8304-1076-8.
- Domino FJ (2010). The 5-Minute Clinical Consult 2011. Lippincott Williams & Wilkins. ISBN 9781608312597.
- Nunn KL, Forney LJ (September 2016). "Unraveling the Dynamics of the Human Vaginal Microbiome". The Yale Journal of Biology and Medicine. 89 (3): 331–337. PMC 5045142. PMID 27698617.
- "NFP Quick Instructions for the Marquette Model (Mucus Only)". Marquette University. 2018.
- Nunn KL, Wang YY, Harit D, Humphrys MS, Ma B, Cone R, Ravel J, Lai SK (October 2015). "Enhanced Trapping of HIV-1 by Human Cervicovaginal Mucus Is Associated with Lactobacillus crispatus-Dominant Microbiota". mBio. 6 (5): e01084–15. doi:10.1128/mBio.01084-15. PMC 4611035. PMID 26443453.
- Shackelford TK, Pound N (2006). Sperm Competition in Humans: Classic and Contemporary Readings. Taylor & Francis. ISBN 9780387280363.
- Reich O, Fritsch H (October 2014). "The developmental origin of cervical and vaginal epithelium and their clinical consequences: a systematic review". Journal of Lower Genital Tract Disease. 18 (4): 358–60. doi:10.1097/lgt.0000000000000023. PMID 24977630.
- Nauth HF (2014). Gynäkologische Zytodiagnostik (in German) (2nd ed.). Stuttgart: Georg Thieme. p. 23. ISBN 978-3-13-131092-7.
- Aroutcheva A.; Gariti D.; Simon M.; Shott S.; Faro J.; Simoes J. A.; Gurguis A.; Faro S. (2001). "Defense factors of vaginal lactobacilli". Am. J. Obstet. Gynecol. 185 (2): 375–379. doi:10.1067/mob.2001.115867. PMID 11518895.
- Linhares, I. M., P. R. Summers, B. Larsen, P. C. Giraldo, and S. S. Witkin. 2011. Contemporary perspectives on vaginal pH and lactobacilli" Am. J. Obstet. Gynecol. 204:120.e1-120.e5.
- Redondo-Lopez V.; Cook R. L.; Sobel J. D. (1990). "Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora". Rev. Infect. Dis. 12 (5): 856–872. doi:10.1093/clinids/12.5.856.
- "Vaginal Cancer Treatment". National Institutes of Health, National Cancer Institute. 2017. Retrieved February 8, 2018.
- "Vaginal cancer | Vaginal cancer | Cancer Research UK". www.cancerresearchuk.org. Retrieved February 8, 2018.
- "About DES". Centers for Disease Control and Prevention. Retrieved February 8, 2018.
- "Known Health Effects for DES Daughters". Centers for Disease Control and Prevention. Retrieved February 8, 2018.
- Kalampokas E, Kalampokas T, Damaskos C (January 2017). "Primary Vaginal Melanoma, A Rare and Aggressive Entity. A Case Report and Review of the Literature". In Vivo. 31 (1): 133–139. doi:10.21873/invivo.11036. PMC 5354139. PMID 28064232.
- "Vaginal yeast infections fact sheet". womenshealth.gov. December 23, 2014. Archived from the original on 4 March 2015. Retrieved 5 March 2015.
- Sharma H, Tal R, Clark NA, Segars JH (January 2014). "Microbiota and pelvic inflammatory disease". Seminars in Reproductive Medicine. 32 (1): 43–9. doi:10.1055/s-0033-1361822. PMC 4148456. PMID 24390920.
- "Bacterial Vaginosis Symptoms, Treatment, Causes & Remedies". eMedicineHealth. Retrieved 2018-02-08.
- Donders G, Bellen G, Rezeberga D (September 2011). "Aerobic vaginitis in pregnancy". BJOG. 118 (10): 1163–70. doi:10.1111/j.1471-0528.2011.03020.x. PMID 21668769.
- Vulvovaginal atrophy and atrophic vaginitis have been the preferred terms for this condition and cluster of symptoms until recently. These terms are now regarded as inaccurate in describing changes to the whole genitourinary system occurring after menopause. The term atrophic vaginitis suggests that the vaginal is inflamed or infected. Though this may be true, inflammation and infection are not the major components of postmenopausal changes to the vagina after menopause. The former terms do not describe the negative effects on the lower urinary tract which can be the most troubling symptoms of menopause for women.
- Kim HK, Kang SY, Chung YJ, Kim JH, Kim MR (August 2015). "The Recent Review of the Genitourinary Syndrome of Menopause". Journal of Menopausal Medicine. 21 (2): 65–71. doi:10.6118/jmm.2015.21.2.65. PMC 4561742. PMID 26357643.
- Faubion SS, Sood R, Kapoor E (December 2017). "Genitourinary Syndrome of Menopause: Management Strategies for the Clinician". Mayo Clinic Proceedings. 92 (12): 1842–1849. doi:10.1016/j.mayocp.2017.08.019. PMID 29202940.
- Greenemeier, Larry. "Small Comfort: Nanomedicine Able to Penetrate Bodily Defenses". Scientific American. Retrieved 2018-02-17.
- Saravanan M, Asmalash T, Gebrekidan A, Gebreegziabiher D, Araya T, Hilekiros H, Barabadi H, Ramanathan K (February 2018). "Nano-medicine as a newly emerging approach to combat Human Immunodeficiency Virus (HIV)". Pharmaceutical Nanotechnology. 6 (1): 17–27. doi:10.2174/2211738506666180209095710. PMID 29424324.
External links
