Pharmacodynamics of progesterone
The pharmacology of progesterone, a progestogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.[1][2]
Progesterone is a naturally occurring and bioidentical progestogen, or an agonist of the progesterone receptor, the biological target of progestogens like endogenous progesterone.[1] Progesterone also has antimineralocorticoid and inhibitory neurosteroid activity, whereas it appears to have little or no glucocorticoid or antiandrogenic activity and has no androgenic activity.[1] Because of its progestogenic activity, progesterone has functional antiestrogenic effects in certain tissues such as the uterus, cervix, and vagina.[1] In addition, progesterone has antigonadotropic effects due to its progestogenic activity and can inhibit fertility and suppress sex hormone production.[1] Progesterone differs from progestins (synthetic progestogens) like medroxyprogesterone acetate and norethisterone, with implications for pharmacodynamics and pharmacokinetics as well as efficacy, tolerability, and safety.[1]
Progesterone can be taken by mouth, in through the vagina, and by injection into muscle or fat, among other routes.[1] A progesterone vaginal ring and progesterone intrauterine device are also available as pharmaceutical products.[3][4]
Mechanism of action
Progesterone is a progestogen, or an agonist of the nuclear progesterone receptors (PRs), the PR-A, PR-B, and PR-C.[1] In one study, progesterone showed EC50 values of 7.7 nM for the human PR-A and 8.0 nM for the human PR-B.[5] In addition to the PRs, progesterone is an agonist of the membrane progesterone receptors (mPRs), including the mPRα, mPRβ, mPRγ, mPRδ, and mPRϵ.[6][7] It is also a potent antimineralocorticoid (antagonist of the mineralocorticoid receptor (MR)),[8][9] as well as a very weak glucocorticoid (agonist of the glucocorticoid receptor).[10][11] Progesterone does not bind to the androgen receptor (AR) or to the estrogen receptor (ER).[1] In addition to its activity as a steroid hormone, progesterone is a neurosteroid.[12] Specifically, it is an antagonist of the sigma σ1 receptor,[13][14] a negative allosteric modulator of nicotinic acetylcholine receptors,[12] and, via its active metabolites allopregnanolone and pregnanolone, a potent positive allosteric modulator of the GABAA receptor, the major signaling receptor of the inhibitory neurotransmitter γ-aminobutyric acid (GABA).[15]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Progesterone | 50 | 0 | 0 | 10 | 100 | 0 | 36 |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. Sources: [1] | |||||||
| Progestogen | Form | Major brand names | Class | TFD (14 days) | POIC-D (2–3 months) | CIC-D (month) | Duration | |
|---|---|---|---|---|---|---|---|---|
| Algestone acetophenide | Oil solution | Perlutal, Topasel, Yectames | Pregnane | ? | – | 75–150 mg | 100 mg ≈ 14–32 days | |
| Cyproterone acetate | Oil solution | Androcur Depot | Pregnane | ? | – | – | 300 mg ≈ 20 days | |
| Dydrogesteronea | Aqueous suspension | – | Retropregnane | ? | – | – | 100 mg ≈ 16–38 days | |
| Gestonorone caproate | Oil solution | Depostat, Primostat | Norpregnane | 50 mg | – | – | 25–50 mg ≈ 8–13 days | |
| Hydroxyprogesterone acetatea | Aqueous suspension | – | Pregnane | 350 mg | – | – | 150–350 mg ≈ 9–16 days | |
| Hydroxyprogesterone caproate | Oil solution | Delalutin, Proluton, Makena | Pregnane | 250–500 mgb | – | 250–500 mg | 65–500 mg ≈ 5–21 days | |
| Levonorgestrel butanoatea | Aqueous suspension | – | Gonane | ? | – | – | 5–50 mg ≈ 3–6 months | |
| Lynestrenol phenylpropionatea | Oil solution | – | Estrane | ? | – | – | 50–100 mg ≈ 14–30 days | |
| Medroxyprogesterone acetate | Aqueous suspension | Depo-Provera | Pregnane | 50–100 mg | 150 mg | 25 mg | 50–150 mg ≈ 14–50+ days | |
| Megestrol acetate | Aqueous suspension | Mego-E | Pregnane | ? | – | 25 mg | 25 mg ≈ >14 daysc | |
| Norethisterone enanthate | Oil solution | Noristerat, Mesigyna | Estrane | 100–200 mg | 200 mg | 50 mg | 50–200 mg ≈ 11–52 days | |
| Oxogestone phenylpropionatea | Oil solution | – | Norpregnane | ? | – | – | 100 mg ≈ 19–20 days | |
| Progesterone | Oil solution | Progestaject, Gestone, Strone | Pregnane | 200 mgb | – | – | 25–350 mg ≈ 2–6 days | |
| Aqueous suspension | Agolutin Depot | Pregnane | 50–200 mg | – | – | 50–300 mg ≈ 7–14 days | ||
| Note: All by intramuscular or subcutaneous injection. All are synthetic except for P4, which is bioidentical. P4 production during the luteal phase is ~25 (15–50) mg/day. The OID of OHPC is 250 to 500 mg/month. Footnotes: a = Never marketed by this route. b = In divided doses (2 × 125 or 250 mg for OHPC, 10 × 20 mg for P4). c = Half-life is ~14 days. Sources: Main: See template. | ||||||||
Antimineralocorticoid activity
Progesterone is a potent antimineralocorticoid.[8][9] It has 1000% of the affinity of aldosterone, the major endogenous agonist, for the human MR, and 100% of the affinity of aldosterone for the rat MR.[16][1][8] Progesterone produces antimineralocorticoid effects such as natriuresis (excretion of sodium in the urine) at normal physiological concentrations.[9] A 200 mg dose of oral progesterone is considered to be approximately equivalent in antimineralocorticoid effect to a 25 to 50 mg dose of the potent antimineralocorticoid spironolactone, which itself is a derivative of progesterone.[17] The antimineralocorticoid effects of progesterone underlie its ability to lower blood pressure and reduce water and salt retention and its potential application in the treatment of hypertension.[18][1][19] An active metabolite of progesterone, 11-deoxycorticosterone (21-hydroxyprogesterone), is a precursor of aldosterone and has strong mineralocorticoid activity (i.e., is a strong agonist of the MR).[17] However, it is formed in relatively small amounts, and any such effects produced by it are usually outweighed by the antimineralocorticoid activity of progesterone.[17]
Glucocorticoid activity
Progesterone is a partial agonist of the glucocorticoid receptor (GR).[1][10][11][20][21] It has about 35% of the affinity of dexamethasone, a corticosteroid, for the human GR, and about 3 to 11% of the affinity of dexamethasone for the rat GR.[16] However, progesterone appears to show weak or no glucocorticoid activity and no antiglucocorticoid activity in vitro and in animals.[21] Nonetheless, progesterone has been found to upregulate the thrombin receptor in vascular smooth muscle cells in vitro, and this could have clinical relevance in relation to risk of blood clots.[1][22]
| Steroid | Class | TR (↑)a | GR (%)b |
|---|---|---|---|
| Dexamethasone | Corticosteroid | ++ | 100 |
| Ethinylestradiol | Estrogen | – | 0 |
| Etonogestrel | Progestin | + | 14 |
| Gestodene | Progestin | + | 27 |
| Levonorgestrel | Progestin | – | 1 |
| Medroxyprogesterone acetate | Progestin | + | 29 |
| Norethisterone | Progestin | – | 0 |
| Norgestimate | Progestin | – | 1 |
| Progesterone | Progestogen | + | 10 |
| Footnotes: a = Thrombin receptor (TR) upregulation (↑) in vascular smooth muscle cells (VSMCs). b = RBA (%) for the glucocorticoid receptor (GR). Strength: – = No effect. + = Pronounced effect. ++ = Strong effect. Sources: See template. | |||
Androgenic and antiandrogenic activities
The binding and activity of progesterone at the AR, the biological target of androgens like testosterone and dihydrotestosterone (DHT), is controversial.[23] Some studies have found progesterone to bind to the AR, with agonistic and antagonistic activity, whereas others have found very low affinity or no affinity at all.[23] In animal studies, no androgenic effects have been observed, but weak antiandrogenic effects have been noticed.[23] This has been attributed not to antagonism of the AR but rather to weak 5α-reductase inhibition and consequent inhibition of the conversion of testosterone into the more potent DHT.[23] There is no clinical evidence of AR-mediated androgenic or antiandrogenic activity of progesterone.[23] As such, the scientific consensus is that progesterone is clinically neither androgenic nor antiandrogenic.[1][24][25] This is in contrast to many progestins, such as 19-nortestosterone derivatives (e.g., norethisterone, levonorgestrel, dienogest) and 17α-hydroxyprogesterone derivatives (e.g., cyproterone acetate, medroxyprogesterone acetate), which do bind to the AR and have been associated with androgenic or antiandrogenic effects depending on the progestin in question.[1][25]
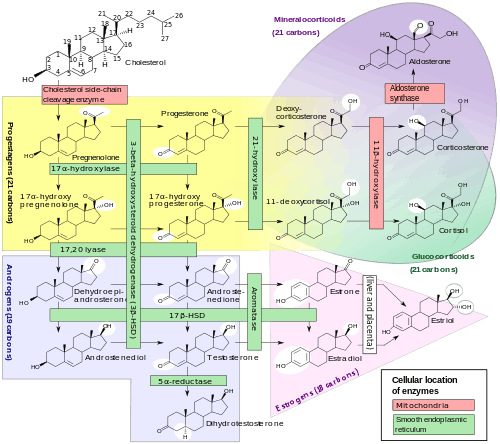
Although progesterone does not have significant AR-mediated androgenic or antiandrogenic activity, it is a precursor and intermediate, albeit distant, in the biosynthesis of androgens from cholesterol.[26][27] For this reason, there has been some speculation that exogenous progesterone could be transformed into androgens by certain tissues that express the requisite enzymes.[27][28] Progesterone is converted by 17α-hydroxylase into 17α-hydroxyprogesterone; 17α-hydroxyprogesterone is converted by 17,20-lyase into androstenedione; and androstenedione is converted by 17β-hydroxysteroid dehydrogenases into testosterone.[26] CYP17A1, the cytochrome P450 gene that encodes 17α-hydroxylase and 17,20-lyase, is expressed mainly in the gonads (ovaries and testes) and the adrenal glands.[29]
However, while it is theoretically possible that progesterone could be transformed in the body into androgens, no androgenic effects have been observed in animal studies.[23] In addition, clinical studies, in which women were treated with 100 to 300 mg/day oral progesterone, have found no or only a small increase in levels of 17α-hydroxyprogesterone, and no change in androgen levels, including of dehydroepiandrosterone, androstenedione, and testosterone.[30][31][32] In these studies, levels of estradiol and cortisol, which progesterone is also a precursor of, did not change either, although levels of 11-deoxycorticosterone increased significantly.[31][32] In accordance with the lack of changes in androgen levels, progesterone, unlike various progestins,[1] has not been associated with androgenic effects in clinical studies, including changes in the blood lipid profile or sex hormone-binding globulin levels,[33][30] acne, skin oiliness, hirsutism, or voice deepening, or induction of teratogenicity (i.e., virilization of female fetuses).[32][34][35]
5α-Reductase inhibition
Progesterone is a substrate for 5α-reductase, and has been found to act as a competitive inhibitor of this enzyme in vitro in a variety of studies.[1] In one study, it showed IC50 values of 1,375 nM and 88 nM (in the presence of 50 nM androstenedione as the substrate) for 5α-reductase types 1 and 2, respectively.[36] 5α-Reductase is highly expressed in skin, hair follicles, and prostate gland, and is responsible for the transformation of testosterone into the several-fold more potent androgen DHT in such tissues.[37][38] As such, it has been suggested that progesterone might possess some antiandrogenic effect via acting as a 5α-reductase inhibitor.[1] However, inhibition of 5α-reductase by progesterone is described as a weak effect that has only been demonstrated in vitro and at supraphysiological concentrations.[39][40] In accordance, physiological levels of circulating progesterone have not been found to importantly influence circulating DHT concentrations.[41][42]
Congenital 5α-reductase 2 deficiency is a rare intersex condition which is associated with ambiguous genitalia in male fetuses due to a deficiency in DHT production during genital differentiation.[38] Experimental prenatal exposure to established 5α-reductase inhibitors like finasteride has been found to produce similar feminized genital defects in male animals including rodents and monkeys.[43] In contrast, exogenous administration of progesterone to pregnant rodents has been found not to produce fetal defects in either male or female pups.[44][45][46] In addition, endogenous progesterone levels naturally increase to extremely high concentrations during pregnancy, yet genital defects do not occur.[47] In accordance, while total concentrations of progesterone in pregnant women at term are around 150 ng/mL (~500 nM), free or unbound and hence bioactive concentrations of progesterone are only about 3 ng/mL (~10 nM) due to the high plasma protein binding of progesterone, and these concentrations are still well below the aforementioned IC50 values for inhibition of 5α-reductase types 1 and 2.[48][49] As with endogenous progesterone during pregnancy, exogenous administration of additional progesterone during pregnancy has been found not to increase the risk of hypospadias in male infants.[50]
Although systemic progesterone does not appear to be an effective 5α-reductase inhibitor, topical progesterone may produce potent inhibition of 5α-reductase in the skin due to the very high local concentrations that occur.[51][52] A study found that topical progesterone applied to the pubic area in men inhibited 5α-reductase in the skin in this region by 75%.[52][53] In addition to inhibition of 5α-reductase, progesterone is metabolized by 5α-reductase into 5α-dihydroprogesterone (5α-DHP), a compound reported to have some antagonistic activity at the AR.[53][54] However, the effectiveness of topical progesterone in pattern hair loss has been poor.[54][55]
Effects in the body and brain
The PRs are expressed widely throughout the body, including in the uterus, cervix, vagina, fallopian tubes, breasts, fat, skin, pituitary gland, hypothalamus, and elsewhere throughout the brain.[1][56] Through activation of the PRs (as well as the mPRs), progesterone has many effects, including the following:[1][56]
- Induces endometrial secretory transformation in preparation for pregnancy
- Prevents estrogen-induced endometrial hyperplasia and increased endometrial cancer risk
- Maintains pregnancy via effects in endometrium (with withdrawal resulting in miscarriage)
- Reduces amount and fibrosity of cervical mucus and causes cervix to become firmer and more tightly closed[57]
- Controls motility and composition of fluid in the fallopian tubes
- Reduced cornification and maturation of the vaginal lining[58]
- Causes water retention in the breasts resulting in temporary enlargement during the menstrual cycle[59][60]
- Mediates lobuloalveolar development of the breasts necessary for lactation
- Suppresses lactation initiation and triggers lactation upon withdrawal (as with parturition)
- Maintains skin health, integrity, appearance, and hydration and slows the rate of aging of the skin[61][62]
- Modulates brain function, with effects on mood, emotionality, and sexuality, as well as cognition and memory
- Exerts negative feedback on the hypothalamic–pituitary–gonadal axis (HPG axis) by suppressing the secretion of the gonadotropins FSH and LH from the pituitary gland (including the mid-cycle gonadotropin surge), thereby inhibiting gonadal sex hormone production as well as ovulation and fertility (>2 ng/mL)[63]
- Increases basal body temperature (by 0.3–0.6 °C (0.5–1.0 °F) relative to preovulation) via the hypothalamus (>4 ng/mL)[64][65]
- Reduces hot flashes via the hypothalamus[66][67]
- Stimulates respiration via the hypothalamus and/or respiratory center[68][69]
- Influences the risk and/or progression of hormone-sensitive cancers including breast cancer and endometrial cancer
Many of the effects of progesterone require estrogen, as estrogens prime tissues for progesterone by inducing expression of the PRs.[1][56] The PRs are induced in the breasts by estrogens, and for this reason, it is assumed that progestogens cannot mediate breast changes in the absence of estrogens.[70]
Progesterone also lowers blood pressure and reduces water and salt retention among other effects via its antimineralocorticoid activity.[1][19]
Progesterone can produce sedative, hypnotic, anxiolytic, euphoric, cognitive-, memory-, and motor-impairing, anticonvulsant, and even anesthetic effects via formation of sufficiently high concentrations of its neurosteroid metabolites and consequent GABAA receptor potentiation in the brain.[18][71][72][73]
Antiestrogenic effects
Progesterone, like all progestogens, has antiestrogenic effects in certain tissues such as the uterus, cervix, and vagina and possibly also the breasts and brain.[1][74][75] These effects are mediated by activation of the PR in these tissues.[1] Progesterone does not have antiestrogenic effects in the more conventional sense of binding to and antagonizing the ER or binding to and inhibiting enzymes involved in estrogen biosynthesis.[1] Instead, for instance in the endometrium, progesterone causes downregulation of the ER and upregulation of the estrogen-inactivating enzymes 17β-hydroxysteroid dehydrogenase 2 (converts estradiol into estrone) and estrone sulfotransferase (converts estrone into estrone sulfate).[1] In the breasts, progesterone similarly downregulates the ER as well as the estrogen-activating enzymes steroid sulfatase (converts estrone sulfate into estrone) and 17β-hydroxysteroid-dehydrogenase 1 (converts estrone into estradiol) and upregulates estrone sulfotransferase.[74][75] The antiestrogenic effects of progesterone and other progestogens form the basis for their only approved indication in menopausal hormone therapy: prevention of long-term unopposed estrogen-induced endometrial hyperplasia and increased endometrial cancer risk in women with intact uteruses.[1]
It has been hypothesized that progestogens may counteract various effects of estrogens in the brain such as stimulatory and excitatory effects on neuronal activity.[1] Progesterone moreover has a special position among progestogens concerning such actions due to its inhibitory neurosteroid metabolites and their central depressant effects.[1] It has been suggested that these actions of progestogens may explain the unfavorable effects on mood that have been observed with these medications in some women.[1] However, the mutual interactions of estrogens and progestogens in the brain in general are controversial and require more research.[1]
Progesterone can also have body-wide antiestrogenic effects at very high doses in both women and men via its antigonadotropic effects and consequent suppression of gonadal estrogen production (see below).[1][76] These antigonadotropic effects are mediated by hyperactivation of the PR.[1][76]
Effects on the HPG axis
Antigonadotropic effects
Progestogens have antigonadotropic effects at sufficiently high doses via activation of the PR and consequent negative feedback on and hence suppression of the hypothalamic–pituitary–gonadal axis (HPG axis).[76] This results in suppression of gonadotropin secretion and by extension interference with fertility and gonadal sex hormone production.[76] Progesterone prevents ovulation by suppressing the mid-cycle surge in gonadotropin secretion during the menstrual cycle.[77][63]
The ovulation-inhibiting (i.e., contraceptive) dosage of oral crystalline (non-micronized) progesterone in women is 300 mg/day or greater.[46][78][1][79] However, this figure is based on limited clinical data.[46] In the clinical research in the 1950s that determined this dosage, ovulation inhibition occurred in 50 to 100% of women when assessed via measures including urinary pregnanediol excretion, daily basal body temperatures, endometrial biopsies, and vaginal smears.[78][80][81][82][83] Another study found that ovulation inhibition with 300 mg/day oral non-micronized progesterone occurred in a "proportion of the cases" when assessed via laparotomy.[82] A third study found that ovulation was inhibited in only 38% of women treated with 1,000 mg/day oral non-micronized progesterone.[79]
In a study of a progesterone vaginal ring alone or in combination with estradiol that released 1.5 to 3 mg/day progesterone and achieved mean progesterone levels varying between 0.7 and 1.6 ng/mL (mean 0.9 ng/mL) during anovulatory cycles, ovulation occurred in 18 of 30 (60%) menstrual cycles.[84] A study of a vaginal progesterone ring that released almost 10 mg/day progesterone and maintained mean progesterone levels of 4.4 ng/mL (range 2.4–6.5 ng/mL) found that ovulation was inhibited in some but not all women.[85][86] In another study, a progesterone vaginal ring that released about 10 mg/day progesterone and produced progesterone levels of around 4 ng/mL (range 3–5.2 ng/mL) resulted in ovulation occurring in 25% of treated breastfeeding women compared to a rate of 56% in a control group of breastfeeding women.[87] A dose of progesterone of 5 to 10 mg/day by intramuscular injection has been found to prevent ovulation in women and hence is effective as a progestogen-only injectable contraceptive.[88][89]
Short-term therapy with 300 mg/day oral progesterone had no effect on luteinizing hormone pulse frequency in women.[90] Treatment with a high dosage of oral progesterone of 100 mg four times per day (or 400 mg/day total) in men for 10 days did not cause any change in testosterone levels, suggesting that progesterone has little or no antigonadotropic effect in males at typical clinical dosages.[18][91] In addition, a study found that administration of 1,000 mg/day oral progesterone for 3 months had no significant effect on urinary gonadotropin excretion.[46] On the other hand, a single 50 mg intramuscular injection of progesterone, which is associated with high progesterone levels of approximately 50 ng/mL (or early- to mid-pregnancy levels),[92][93][94] resulted in substantial (50–60%) suppression of luteinizing hormone, follicle-stimulating hormone, and testosterone levels in men.[95][96] Similarly, continuous or intermittent intravenous injections of 100 to 400 mg/day progesterone for 10 days significantly decreased urinary gonadotropin excretion.[46][97] Progestogens in general are able to suppress gonadal testosterone production in men by a maximum of about 70 to 80% or to just above castrate levels when used at sufficiently high doses.[98][99]
A study using 50 mg/day progesterone by intramuscular injection in five men found that the medication produced azoospermia or severe oligozoospermia in all within 10 weeks, suppressed libido, erectile function, and ejaculate volume to minimal levels, produced slight gynecomastia in two of the men, moderately decreased testicular size, and impaired testicular morphology.[89][100][101][96][102][103][104] Upon discontinuation, sperm counts returned to normal in the men within 14 to 17 weeks.[89][100][96][102][104] In another study, 100 mg rectal suppositories of progesterone given five times per day for 9 days resulted in progesterone levels of 5.5 to 29 ng/mL and suppressed circulating testosterone and growth hormone levels by about 50% in men, but did not affect libido or erectile potency with this short duration of therapy.[89][105]
Progonadotropic effects
Progesterone has progonadotropic effects at certain times during the menstrual cycle.[77]
Neurosteroid effects
Progesterone, through the actions of neurosteroid active metabolites such as allopregnanolone and pregnanolone, is a potent positive allosteric modulator of the GABAA receptor, the major signaling receptor of the inhibitory neurotransmitter γ-aminobutyric acid (GABA).[15] It can produce sedative, hypnotic, anxiolytic, euphoric, cognitive-, memory-, and motor-impairing, anticonvulsant, and even anesthetic effects with formation of sufficiently high concentrations of its neurosteroid metabolites and consequent GABAA receptor potentiation in the brain.[18][71][72][73] These actions and effects are characteristically similar to those of other GABAA receptor positive allosteric modulators like alcohol, barbiturates, and benzodiazepines.[73]
Similarly to other GABAA receptor positive allosteric modulators like alcohol, barbiturates, and benzodiazepines, tolerance has been found to develop with exposure to increased levels of allopregnanolone and related inhibitory neurosteroids.[106][107] This includes downregulation and desensitization of the GABAA receptor, reduced effects of allopregnanolone and other GABAA receptor activators (e.g., GABA and benzodiazepines), and rebound or withdrawal effects upon falls in allopregnanolone levels.[106][107] In addition, changes in allopregnanolone levels have been implicated in adverse neuropsychiatric effects associated with the menstrual cycle (e.g., dysphoria, depression, anxiety, irritability) and postpartum period (e.g., postpartum depression), as well as in catamenial epilepsy (seizures).[108][109] Low and high levels of allopregnanolone seem to have a neutral effect on mood, whereas moderate levels have a negative effect, which may underlie the symptoms of premenstrual syndrome and premenstrual dysphoric disorder that are observed in 30 to 40% of premenopausal women.[108][109][110] This U-shaped effect on mood appears to be a common property of GABAA receptor positive allosteric modulators.[108][109]
See also
References
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Unfer, Vittorio; di Renzo, Gian; Gerli, Sandro; Casini, Maria (2006). "The Use of Progesterone in Clinical Practice: Evaluation of its Efficacy in Diverse Indications Using Different Routes of Administration". Current Drug Therapy. 1 (2): 211–219. doi:10.2174/157488506776930923. ISSN 1574-8855.
- Whitaker, Amy; Gilliam, Melissa (2014). Contraception for Adolescent and Young Adult Women. Springer. p. 98. ISBN 9781461465799.
- Chaudhuri (2007). Practice Of Fertility Control: A Comprehensive Manual (7Th Edition). Elsevier India. pp. 153–. ISBN 978-81-312-1150-2.
- Attardi BJ, Burgenson J, Hild SA, Reel JR (March 2004). "In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone". J. Steroid Biochem. Mol. Biol. 88 (3): 277–88. doi:10.1016/j.jsbmb.2003.12.004. PMID 15120421.
- Soltysik K, Czekaj P (April 2013). "Membrane estrogen receptors - is it an alternative way of estrogen action?". J. Physiol. Pharmacol. 64 (2): 129–42. PMID 23756388.
- Prossnitz ER, Barton M (May 2014). "Estrogen biology: New insights into GPER function and clinical opportunities". Mol. Cell. Endocrinol. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. PMC 4040308. PMID 24530924.
- Rupprecht R, Reul JM, van Steensel B, Spengler D, Söder M, Berning B, Holsboer F, Damm K (October 1993). "Pharmacological and functional characterization of human mineralocorticoid and glucocorticoid receptor ligands". European Journal of Pharmacology. 247 (2): 145–54. doi:10.1016/0922-4106(93)90072-H. PMID 8282004.
- Elger W, Beier S, Pollow K, Garfield R, Shi SQ, Hillisch A (2003). "Conception and pharmacodynamic profile of drospirenone". Steroids. 68 (10–13): 891–905. doi:10.1016/j.steroids.2003.08.008. PMID 14667981.
- Attardi BJ, Zeleznik A, Simhan H, Chiao JP, Mattison DR, Caritis SN (2007). "Comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-alpha hydroxyprogesterone caproate, and related progestins". Am. J. Obstet. Gynecol. 197 (6): 599.e1–7. doi:10.1016/j.ajog.2007.05.024. PMC 2278032. PMID 18060946.
- Lei K, Chen L, Georgiou EX, Sooranna SR, Khanjani S, Brosens JJ, Bennett PR, Johnson MR (2012). "Progesterone acts via the nuclear glucocorticoid receptor to suppress IL-1β-induced COX-2 expression in human term myometrial cells". PLOS ONE. 7 (11): e50167. Bibcode:2012PLoSO...750167L. doi:10.1371/journal.pone.0050167. PMC 3509141. PMID 23209664.
- Baulieu E, Schumacher M (2000). "Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination". Steroids. 65 (10–11): 605–12. doi:10.1016/s0039-128x(00)00173-2. PMID 11108866.
- Maurice T, Urani A, Phan VL, Romieu P (November 2001). "The interaction between neuroactive steroids and the sigma1 receptor function: behavioral consequences and therapeutic opportunities". Brain Research. Brain Research Reviews. 37 (1–3): 116–32. doi:10.1016/s0165-0173(01)00112-6. PMID 11744080.
- Johannessen M, Fontanilla D, Mavlyutov T, Ruoho AE, Jackson MB (February 2011). "Antagonist action of progesterone at σ-receptors in the modulation of voltage-gated sodium channels". American Journal of Physiology. Cell Physiology. 300 (2): C328–37. doi:10.1152/ajpcell.00383.2010. PMC 3043630. PMID 21084640.
- Paul SM, Purdy RH (March 1992). "Neuroactive steroids". FASEB Journal. 6 (6): 2311–22. doi:10.1096/fasebj.6.6.1347506. PMID 1347506.
- Krattenmacher R (July 2000). "Drospirenone: pharmacology and pharmacokinetics of a unique progestogen". Contraception. 62 (1): 29–38. doi:10.1016/S0010-7824(00)00133-5. PMID 11024226.
- Simon JA (December 1995). "Micronized progesterone: vaginal and oral uses". Clinical Obstetrics and Gynecology. 38 (4): 902–14. doi:10.1097/00003081-199538040-00024. PMID 8616985.
- Goletiani NV, Keith DR, Gorsky SJ (2007). "Progesterone: review of safety for clinical studies". Exp Clin Psychopharmacol. 15 (5): 427–44. doi:10.1037/1064-1297.15.5.427. PMID 17924777.
- Oelkers W (2000). "Drospirenone--a new progestogen with antimineralocorticoid activity, resembling natural progesterone". Eur J Contracept Reprod Health Care. 5 Suppl 3: 17–24. PMID 11246598.
- Zerr-Fouineau M, Chataigneau M, Blot C, Schini-Kerth VB (January 2007). "Progestins overcome inhibition of platelet aggregation by endothelial cells by down-regulating endothelial NO synthase via glucocorticoid receptors". FASEB J. 21 (1): 265–73. doi:10.1096/fj.06-6840com. PMID 17116740.
- Fuhrmann U, Krattenmacher R, Slater EP, Fritzemeier KH (October 1996). "The novel progestin drospirenone and its natural counterpart progesterone: biochemical profile and antiandrogenic potential". Contraception. 54 (4): 243–51. doi:10.1016/s0010-7824(96)00195-3. PMID 8922878.
Drospirenone and progesterone exhibited low binding affinities to the rat GR as is documented by 1% and 11% RBA values compared to the reference dexamethasone, respectively. Similar results were reported elsewhere.8 In accordance with the low affinity to the GR, progesterone and drospirenone showed weak or no detectable agonistic activities, respectively, in the GR-dependent transactivation assay (Figure 2A and Figure 2B). Furthermore, both progestins were devoid of antiglucocorticoid activity in vitro. These data are in agreement with in vivo studies carried out with rats where drospirenone and progesterone showed neither glucocorticoid nor antiglucocorticoid activity.8
- Wiegratz I, Kuhl H (August 2004). "Progestogen therapies: differences in clinical effects?". Trends Endocrinol. Metab. 15 (6): 277–85. doi:10.1016/j.tem.2004.06.006. PMID 15358281.
- Yeh YT, Chang CW, Wei RJ, Wang SN (2013). "Progesterone and related compounds in hepatocellular carcinoma: basic and clinical aspects". Biomed Res Int. 2013: 290575. doi:10.1155/2013/290575. PMC 3581253. PMID 23484104.
- Sitruk-Ware R (2002). "Progestogens in hormonal replacement therapy: new molecules, risks, and benefits". Menopause. 9 (1): 6–15. doi:10.1097/00042192-200201000-00003. PMID 11791081.
- Sumino, Hiroyuki; Ichikawa, Shuichi; Kasama, Shu; Takahashi, Takashi; Kumakura, Hisao; Takayama, Yoshiaki; Minami, Kazutomo; Kanda, Tsugiyasu; Kurabayashi, Masahiko; Murakami, Masami (2011). "Hormone Therapy and Blood Pressure in Postmenopausal Women". Journal of Experimental & Clinical Medicine. 3 (3): 112–115. doi:10.1016/j.jecm.2011.04.005. ISSN 1878-3317.
Natural progesterone, such as micronized progesterone, has no androgenic properties, whereas some synthetic progestins, such as MPA and norethisterone acetate, possess androgenic side effects, which raise the concern of potentially harmful effects on blood pressure.
- Häggström, Mikael; Richfield, David (2014). "Diagram of the pathways of human steroidogenesis". WikiJournal of Medicine. 1 (1). doi:10.15347/wjm/2014.005. ISSN 2002-4436.
- Samuel B. Frank (1971). Acne Vulgaris. Thomas. p. 131.
The chemical structure of progesterone and testosterone are remarkably similar; they differ only in the side chain at the 17-carbon position. The possibility that progesterone can be transformed to testosterone has been considered good by many. If true, it could then be a source of androgens in women. [...] Laboratory evidence exists that progesterone can be converted to testosterone in vitro by human and animal ovarian and testicular tissue.44-47 Although the role of progesterone in acne and its effect on sebaceous gland activity is not fully established, the possibility that endogenous progesterone is a precursor of testosterone or of another androgenic substance invites further exploration.48,49
- Vermorken, A. J. M.; Houben, J. J. G. (2016). "Topical Androgen Treatment for ACNE a Review". Drug Intelligence & Clinical Pharmacy. 12 (3): 151–157. doi:10.1177/106002807801200302. ISSN 0012-6578.
The only concern Voigt and Hsia expressed about the use of progesterone as an anti-androgen was the possibility that the small amount of hormone which reached the circulation could be converted into testosterone by the sexual organs, mainly the testes.
- Shufeng Zhou (6 April 2016). Cytochrome P450 2D6: Structure, Function, Regulation and Polymorphism. CRC Press. pp. 52–. ISBN 978-1-4665-9788-4.
- Woods KS, Reyna R, Azziz R (2002). "Effect of oral micronized progesterone on androgen levels in women with polycystic ovary syndrome". Fertil. Steril. 77 (6): 1125–7. doi:10.1016/s0015-0282(02)03119-9. PMID 12057716.
The mean values of TT, FT, SHBG, DHEAS, A4, and 17-OHP did not change with OMP administration. However, a higher 17-OHP level was observable at the completion of OMP administration (week 2).
- Whitehead MI, Townsend PT, Gill DK, Collins WP, Campbell S (1980). "Absorption and metabolism of oral progesterone". Br Med J. 280 (6217): 825–7. doi:10.1136/bmj.280.6217.825. PMC 1600943. PMID 7370683.
Plasma concentrations of oestradiol were unchanged by giving progesterone.
- Ottosson UB (1984). "Oral progesterone and estrogen/progestogen therapy. Effects of natural and synthetic hormones on subfractions of HDL cholesterol and liver proteins". Acta Obstet Gynecol Scand Suppl. 127: 1–37. doi:10.3109/00016348409157016. PMID 6596830.
Natural progesterone is devoid of any androgenic activity that might compromise lipoprotein metabolism or induce teratogenicity.
- Samsioe, Göran; Dören, Martina; Lobo, Rogerio A (2006). "Hormone replacement therapy – the agents". Women's Health Medicine. 3 (5): 213–216. doi:10.1053/S1744-1870(06)70207-4. ISSN 1744-1870.
Progestogens differ in their relative metabolic and androgenic effects; for example MPA is minimally androgenic, but does counteract the rise in HDL-cholesterol caused by oestrogen therapy. In contrast, oral micronized progesterone does not mitigate against increased HDL-cholesterol levels.
- Zutshi (2005). Hormones in Obstetrics and Gynaecology. Jaypee Brothers, Medical Publishers. pp. 74–75. ISBN 978-81-8061-427-9.
It has been observed that micronized progesterone has no suppressive effects on high-density lipoprotein-cholesterol (HDL-C). Jensen et al have proved that oral micronized progesterone has no adverse effect on serum lipids. These preparations have the same antiestrogenic and antimineralocorticoid effect but no androgenic action. It does not affect aldosterone synthesis, blood pressure, carbohydrate metabolism or mood changes. No side effects have been reported as far as lipid profile, coagulation factors and blood pressure are concerned.
- Levy T, Yairi Y, Bar-Hava I, Shalev J, Orvieto R, Ben-Rafael Z (2000). "Pharmacokinetics of the progesterone-containing vaginal tablet and its use in assisted reproduction" (PDF). Steroids. 65 (10–11): 645–9. doi:10.1016/s0039-128x(00)00121-5. PMID 11108871.
Natural progesterone is devoid of any androgenic activity and is thus extensively used in assisted reproduction, sometimes for long periods of time.
- Rižner TL, Brožič P, Doucette C, Turek-Etienne T, Müller-Vieira U, Sonneveld E, van der Burg B, Böcker C, Husen B (May 2011). "Selectivity and potency of the retroprogesterone dydrogesterone in vitro". Steroids. 76 (6): 607–15. doi:10.1016/j.steroids.2011.02.043. PMID 21376746.
- Swerdloff RS, Dudley RE, Page ST, Wang C, Salameh WA (2017). "Dihydrotestosterone: Biochemistry, Physiology, and Clinical Implications of Elevated Blood Levels". Endocr. Rev. 38 (3): 220–254. doi:10.1210/er.2016-1067. PMC 6459338. PMID 28472278.
- Marks LS (2004). "5α-reductase: history and clinical importance". Rev Urol. 6 Suppl 9: S11–21. PMC 1472916. PMID 16985920.
- Golub MS, Kaufman FL, Campbell MA, Li LH, Donald JM (October 2006). ""Natural" progesterone: information on fetal effects". Birth Defects Res. B Dev. Reprod. Toxicol. 77 (5): 455–70. doi:10.1002/bdrb.20089. PMID 17066418.
Progesterone has been shown to inhibit 5α-reductase, another important enzyme in steroid hormone metabolism, (Dean and Winter, 1984; Beckmann et al., 1993; Cassidenti et al., 1991; Kadohama et al., 1983; Mauvais-Jarvis et al., 1974; Dube et al., 1975). However, this is a weak effect that has only been demonstrated at supra-physiological concentrations and in vitro conditions.
- Kincl, Fred A. (1990). "Control of Reproductive Function in the Adult". Hormone Toxicity in the Newborn. Monographs on Endocrinology. 31. pp. 5–120. doi:10.1007/978-3-642-83794-4_2. ISBN 978-3-642-83796-8. ISSN 0077-1015.
Progesterone (and other progestational agents) inhibit testosterone from expressing its activity at the target sites (Kincl, 1971a). Mice and rats are the test animals of choice (Dorfman, 1963a,b). Inhibition of 5α-reductase activity of binding to cytosol and nuclear receptors has been shown to be the steps at which antiandrogens express their activity (Neumann and Steinbeck, 1974). Relatively high amounts are needed to achieve a significant effect (Table 2.16).
- Dewis P, Newman M, Anderson DC (October 1984). "The effect of endogenous progesterone on serum levels of 5α-reduced androgens in hirsute women". Clin. Endocrinol. (Oxf). 21 (4): 383–92. doi:10.1111/j.1365-2265.1984.tb03225.x. PMID 6542470.
These studies suggest that [...] a rise in serum progesterone has only a minimal effect on circulating levels of the active 5α‐reduced androgen metabolites. [...] Progesterone has been shown to be a potent in vitro inhibitor of cutaneous 5α-reductase (Mauvais-Jarvis et al., 1974). However we found only a small reduction in serum DHT levels in the late luteal phase in ovulatory women and no change in serum 3α-diol. Hence the rise in serum progesterone in ovulatory women has only a minimal effect on the circulating levels of the major active 5α-reduced androgens in vivo.
- Kålund-Jensen H, Myrén CJ (December 1984). "Vaginal absorption of oestradiol and progesterone". Maturitas. 6 (4): 359–67. doi:10.1016/0378-5122(84)90009-4. PMID 6543461.
- Picut CA, Ziejewski MK, Stanislaus D (February 2018). "Comparative Aspects of Pre- and Postnatal Development of the Male Reproductive System". Birth Defects Res. 110 (3): 190–227. doi:10.1002/bdr2.1133. PMID 29063715.
- Fred A. Kincl (6 December 2012). Hormone Toxicity in the Newborn. Springer Science & Business Media. p. 60. ISBN 978-3-642-83794-4.
- Kawashima K, Nakaura S, Nagao S, Tanaka S, Kuwamura T (February 1977). "Virilizing activities of various steroids in female rat fetuses". Endocrinol. Jpn. 24 (1): 77–81. doi:10.1507/endocrj1954.24.77. PMID 558879.
- Aufrère MB, Benson H (June 1976). "Progesterone: an overview and recent advances". J Pharm Sci. 65 (6): 783–800. doi:10.1002/jps.2600650602. PMID 945344.
Early studies on its use as an oral contraceptive showed that, at 300 mg/day (5th to 25th day of the menstrual cycle), progesterone was effective in preventing ovulation through four cycles (263). The related effect of larger doses of progesterone on gonadotropin excretion also has been investigated. Rothchild (264) found that continuous or intermittent intravenously administered progesterone (100-400 mg/day) for 10 days depressed the total amount of gonadotropin excreted into the urine. However, Paulsen et al. (265) found that oral progesterone at 1000 mg/day for 87 days did not have a significant effect on urinary gonadotropin excretion. The efficacy of progesterone as an oral contraceptive was never fully tested, because synthetic progestational agents, which were orally effective, were available.
- Tony M. Plant; Anthony J. Zeleznik (15 November 2014). Knobil and Neill's Physiology of Reproduction. Academic Press. pp. 2289, 2386. ISBN 978-0-12-397769-4.
- Hormones, Brain and Behavior, Five-Volume Set. Elsevier. 18 June 2002. pp. 54–. ISBN 978-0-08-053415-2.
- Heidrich A, Schleyer M, Spingler H, Albert P, Knoche M, Fritze J, Lanczik M (February 1994). "Postpartum blues: relationship between not-protein bound steroid hormones in plasma and postpartum mood changes". J Affect Disord. 30 (2): 93–8. doi:10.1016/0165-0327(94)90036-1. PMID 8201129.
- Baek, K.; Rosenwaks, Z.; Poppas, D.P.; Palermo, G.D. (2006). "P-657". Fertility and Sterility. 86 (3): S377. doi:10.1016/j.fertnstert.2006.07.1033. ISSN 0015-0282.
- Pharmacology of the Skin I: Pharmacology of Skin Systems Autocoids in Normal and Inflamed Skin. Springer Science & Business Media. 6 December 2012. pp. 249–250. ISBN 978-3-642-73797-8.
- Pharmacology of the Skin II: Methods, Absorption, Metabolism and Toxicity, Drugs and Diseases. Springer Science & Business Media. 6 December 2012. pp. 253, 485–. ISBN 978-3-642-74054-1.
- Walter P. Unger (1 February 1995). "Androgenetic alopecia and its treatment. A historical overview". Hair Transplantation, Third Edition. Taylor & Francis. pp. 1–33. ISBN 978-0-8247-9363-0.
- Sawaya, Marty E.; Shapiro, Jerry (2000). "Androgenetic alopecia". Dermatologic Clinics. 18 (1): 47–61. doi:10.1016/S0733-8635(05)70146-7. ISSN 0733-8635. PMID 10626111.
- Price, Vera H. (1988). "Androgenetic alopecia and hair growth promotion state of the art: Present and future". Clinics in Dermatology. 6 (4): 218–227. doi:10.1016/0738-081X(88)90090-9. ISSN 0738-081X.
- P. J. Bentley (1980). Endocrine Pharmacology: Physiological Basis and Therapeutic Applications. CUP Archive. pp. 264, 274. ISBN 978-0-521-22673-8.
- Sue Macdonald; Gail Johnson (3 June 2017). Mayes' Midwifery E-Book. Elsevier Health Sciences. pp. 391–. ISBN 978-0-7020-6336-7.
- Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 889, 940. ISBN 978-0-7817-1750-2.
- Lee-Ellen C. Copstead-Kirkhorn; Jacquelyn L. Banasik (25 June 2014). Pathophysiology - E-Book. Elsevier Health Sciences. pp. 660–. ISBN 978-0-323-29317-4.
Throughout the reproductive years, some women note swelling of the breast around the latter part of each menstrual cycle before the onset of menstruation. The water retention and subsequent swelling of breast tissue during this phase of the menstrual cycle are thought to be due to high levels of circulating progesterone stimulating the secretory cells of the breast.12
- Farage MA, Neill S, MacLean AB (2009). "Physiological changes associated with the menstrual cycle: a review". Obstet Gynecol Surv. 64 (1): 58–72. doi:10.1097/OGX.0b013e3181932a37. PMID 19099613.
- Raine-Fenning NJ, Brincat MP, Muscat-Baron Y (2003). "Skin aging and menopause : implications for treatment". Am J Clin Dermatol. 4 (6): 371–8. doi:10.2165/00128071-200304060-00001. PMID 12762829.
- Holzer G, Riegler E, Hönigsmann H, Farokhnia S, Schmidt JB, Schmidt B (2005). "Effects and side-effects of 2% progesterone cream on the skin of peri- and postmenopausal women: results from a double-blind, vehicle-controlled, randomized study". Br. J. Dermatol. 153 (3): 626–34. doi:10.1111/j.1365-2133.2005.06685.x. PMID 16120154.
- Leon Speroff; Marc A. Fritz (2005). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 211–. ISBN 978-0-7817-4795-0.
When administered before the estrogen stimulus, or in high doses (achieving a blood level greater than 2 ng/mL), progesterone blocks the midcycle LH surge.
- Charles R. B. Beckmann; William Herbert; Douglas Laube; Frank Ling, Roger Smith (21 January 2013). Obstetrics and Gynecology. Lippincott Williams & Wilkins. pp. 342–. ISBN 978-1-4698-2604-2.
- Quigley MM (August 1986). "Drugs in the treatment of female infertility. Recent advances". Drugs. 32 (2): 169–77. doi:10.2165/00003495-198632020-00004. PMID 3527660.
In the presence of circulating levels of approximately 4 μg/L or greater of progesterone, most women experience a 0.5° to 1°F rise in basal body temperature.
- Shanafelt TD, Barton DL, Adjei AA, Loprinzi CL (2002). "Pathophysiology and treatment of hot flashes". Mayo Clin. Proc. 77 (11): 1207–18. doi:10.4065/77.11.1207. PMID 12440557.
- Sassarini J, Lumsden MA (2010). "Hot flushes: are there effective alternatives to estrogen?". Menopause Int. 16 (2): 81–8. doi:10.1258/mi.2010.010007. PMID 20729500.
- Bayliss DA, Millhorn DE (1992). "Central neural mechanisms of progesterone action: application to the respiratory system". J. Appl. Physiol. 73 (2): 393–404. doi:10.1152/jappl.1992.73.2.393. PMID 1399957.
- Ghada Bourjeily; Karen Rosene-Montella (21 April 2009). Pulmonary Problems in Pregnancy. Springer Science & Business Media. pp. 21–. ISBN 978-1-59745-445-2.
- Gompel A, Plu-Bureau G (August 2018). "Progesterone, progestins and the breast in menopause treatment". Climacteric. 21 (4): 326–332. doi:10.1080/13697137.2018.1476483. PMID 29852797.
- Wang-Cheng R, Neuner JM, Barnabei VM (2007). Menopause. ACP Press. p. 97. ISBN 978-1-930513-83-9.
- Bergemann N, Ariecher-Rössler A (27 December 2005). Estrogen Effects in Psychiatric Disorders. Springer Science & Business Media. p. 179. ISBN 978-3-211-27063-9.
- Bäckström T, Bixo M, Johansson M, Nyberg S, Ossewaarde L, Ragagnin G, Savic I, Strömberg J, Timby E, van Broekhoven F, van Wingen G (2014). "Allopregnanolone and mood disorders". Prog. Neurobiol. 113: 88–94. doi:10.1016/j.pneurobio.2013.07.005. PMID 23978486.
- Pasqualini JR (2007). "Progestins and breast cancer". Gynecol. Endocrinol. 23 Suppl 1: 32–41. doi:10.1080/09513590701585003. PMID 17943537.
- Pasqualini JR (2009). "Breast cancer and steroid metabolizing enzymes: the role of progestogens". Maturitas. 65 Suppl 1: S17–21. doi:10.1016/j.maturitas.2009.11.006. PMID 19962254.
- de Lignières B, Silberstein S (April 2000). "Pharmacodynamics of oestrogens and progestogens". Cephalalgia: An International Journal of Headache. 20 (3): 200–7. doi:10.1046/j.1468-2982.2000.00042.x. PMID 10997774.
- Shaw RW (November 1978). "Neuroendocrinology of the menstrual cycle in humans". Clin Endocrinol Metab. 7 (3): 531–59. doi:10.1016/S0300-595X(78)80008-5. PMID 365398.
- Endrikat J, Gerlinger C, Richard S, Rosenbaum P, Düsterberg B (December 2011). "Ovulation inhibition doses of progestins: a systematic review of the available literature and of marketed preparations worldwide". Contraception. 84 (6): 549–57. doi:10.1016/j.contraception.2011.04.009. PMID 22078182.
Table 1 Publications on ovulation inhibition doses of progestins: Progestin: Progesterone. Reference: Pincus (1956). Method: Urinary pregnanediol. Daily dose (mg): 300.000. Total number of cycles in all subjects: 61. Total number of ovulation in all subjects: 30. % of ovulation in all subjects: 49.
- Stone, Abraham; Kupperman, Herbert S. (1955). "The Effects of Progesterone on Ovulation: A Preliminary Report". The Fifth International Conference on Planned Parenthood: Theme, Overpopulation and Family Planning: Report of the Proceedings, 24-29 October, 1955, Tokyo, Japan. International Planned Parenthood Federation. p. 185.
The results of testing the effects of progesterone on ovulation in 13 patients at the Margaret Sanger Research Bureau are presented. The patients had normal menstrual cycles and showed clear evidence of ovulation. Each patient was given 1000 [mg] of [oral] progesterone daily during the midperiod for 10 or 12 days during 16 cycles. Ovulation was inhibited in 6 cycles. No disturbance in menstrual rhythm was observed. 3 of 12 patients with longstanding infertility histories became pregnant within 2-4 months after the cessation of progesterone therapy.
- Pincus G (1956). "Some effects of progesterone and related compounds upon reproduction and early development in mammals". Acta Endocrinol Suppl (Copenh). 23 (Suppl 28): 18–36. doi:10.1530/acta.0.023S018. PMID 13394044.
- Pincus G (December 1958). "The hormonal control of ovulation and early development". Postgrad Med. 24 (6): 654–60. doi:10.1080/00325481.1958.11692305. PMID 13614060.
Table 1: Effects of oral progesterone on three indexes of ovulation: Medication: Progesterone. Number: 69. Mean cycle length: 25.5 ± 0.59. Per cent positive for ovulation by: Basal temperature: 27. Endometrial biopsy: 18. Vaginal smear: 6. [...] we settled on 300 mg. per day [oral progersterone] as a significantly effective [ovulation inhibition] dosage, and this was administered from the fifth day through the twenty-fourth day of the menstrual cycle. [...] We observed each of 33 volunteer subjects during a control, nontreatment cycle and for one to three successive cycles of medication immediately following the control cycle. As indexes of the occurrence of ovulation, daily basal temperatures and vaginal smears were taken, and at the nineteenth to twenty-second day of the cycle an endometrial biopsy. [...] Although we thus demonstrated the ovulation-inhibiting activity of progesterone in normally ovulating women, oral progesterone medication had two disadvantages: ( l) the large daily dosage ( 300 mg.) which presumably would have to be even larger if one sought 100 per cent inhibition1 [...]
- Pincus, Gregory (1959). Progestational Agents and the Control of Fertility. Vitamins & Hormones. 17. pp. 307–324. doi:10.1016/S0083-6729(08)60274-5. ISBN 9780127098173. ISSN 0083-6729.
Ishikawa et al. (1957) employing the same regime of progesterone administration also observed suppression of ovulation in a proportion of the cases taken to laparotomy. Although sexual intercourse was practised freely by the subjects of our experiments and those of Ishikawa el al., no pregnancies OCcurred. Since ovulation presumably took place in a proportion of cycles, the lack of any pregnancies may be due to chance, but Ishikawa et al. (1957) have presented data indicating that in women receiving oral progesterone the cervical mucus becomes impenetrable to sperm.
- Rock J, Garcia CR, Pincus G (1957). "Synthetic progestins in the normal human menstrual cycle". Recent Prog. Horm. Res. 13: 323–39, discussion 339–46. PMID 13477811.
- Victor A, Jackanicz TM, Johansson ED (December 1978). "Vaginal progesterone for contraception". Fertil. Steril. 30 (6): 631–5. doi:10.1016/S0015-0282(16)43688-5. PMID 729823.
- Croxatto HB, Díaz S (1987). "The place of progesterone in human contraception". J. Steroid Biochem. 27 (4–6): 991–4. doi:10.1016/0022-4731(87)90179-8. PMID 3320572.
- Bäckström T, von Schoultz B, Toivonen J (1979). "Plasma progesterone concentrations after administration via intravaginal rings". Acta Obstet Gynecol Scand. 58 (2): 211–2. doi:10.3109/00016347909154585. PMID 452876.
- Shaaban MM (1991). "Contraception with progestogens and progesterone during lactation". J. Steroid Biochem. Mol. Biol. 40 (4–6): 705–10. doi:10.1016/0960-0760(91)90294-F. PMID 1835650.
- Netter A, Gorins A, Thomas K, Cohen M, Joubinaux J (1973). "Blocage du pic d'ovulation de LH et de FSH par la progesterone à faibles doses chez la femme" [Blockade of LH and FSH peaks by low doses of exogenous progesterone in the human female]. Ann. Endocrinol. (Paris) (in French). 34 (4): 430–5. ISSN 0003-4266. PMID 4779738.
- Janet Brotherton (1976). Sex Hormone Pharmacology. Academic Press. pp. 220, 341–342. ISBN 978-0-12-137250-7.
- Lobo, Rogerio A.; Stanczyk, Frank Z. (1994). "New knowledge in the physiology of hormonal contraceptives". American Journal of Obstetrics and Gynecology. 170 (5): 1499–1507. doi:10.1016/S0002-9378(12)91807-4. ISSN 0002-9378.
- Tollan A, Oian P, Kjeldsen SE, Eide I, Maltau JM (1993). "Progesterone reduces sympathetic tone without changing blood pressure or fluid balance in men". Gynecol. Obstet. Invest. 36 (4): 234–8. doi:10.1159/000292636. PMID 8300009.
- Progesterone - Drugs.com, retrieved 2015-08-23
- Josimovich J (11 November 2013). Gynecologic Endocrinology. Springer Science & Business Media. pp. 9, 25–29, 139. ISBN 978-1-4613-2157-6.
- Jerome Frank Strauss; Robert L. Barbieri (2009). Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Elsevier Health Sciences. pp. 807–. ISBN 978-1-4160-4907-4.
- Brady BM, Anderson RA, Kinniburgh D, Baird DT (2003). "Demonstration of progesterone receptor-mediated gonadotrophin suppression in the human male". Clin. Endocrinol. (Oxf). 58 (4): 506–12. doi:10.1046/j.1365-2265.2003.01751.x. PMID 12641635.
- Heller CG, Moore DJ, Paulsen CA, Nelson WO, Laidlaw WM (December 1959). "Effects of progesterone and synthetic progestins on the reproductive physiology of normal men". Fed. Proc. 18: 1057–65. PMID 14400846.
- Rothchild I (June 1957). "Effect of large doses of intravenously administered progesterone on gonadotropin excretion in the human female". J. Clin. Endocrinol. Metab. 17 (6): 754–9. doi:10.1210/jcem-17-6-754. PMID 13428841.
- Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (25 August 2011). Campbell-Walsh Urology: Expert Consult Premium Edition: Enhanced Online Features and Print, 4-Volume Set. Elsevier Health Sciences. pp. 2938–. ISBN 978-1-4160-6911-9.
- Kjeld JM, Puah CM, Kaufman B, Loizou S, Vlotides J, Gwee HM, Kahn F, Sood R, Joplin GF (1979). "Effects of norgestrel and ethinyloestradiol ingestion on serum levels of sex hormones and gonadotrophins in men". Clinical Endocrinology. 11 (5): 497–504. doi:10.1111/j.1365-2265.1979.tb03102.x. PMID 519881.
- Neumann, F.; Diallo, F.A.; Hasan, S.H.; Schenck, B.; Traore, I. (1976). "The Influence of Pharmaceutical Compounds on Male Fertility*". Andrologia. 8 (3): 203–235. doi:10.1111/j.1439-0272.1976.tb02137.x. ISSN 0303-4569. PMID 793446.
- Heller CG, Laidlaw WM, Harvey HT, Nelson WO (July 1958). "Effects of progestational compounds on the reproductive processes of the human male". Ann. N. Y. Acad. Sci. 71 (5): 649–65. doi:10.1111/j.1749-6632.1958.tb54641.x. PMID 13583821.
- Neumann, F. (1985). "Steroidal contraception — experimental background". Future Aspects in Contraception: 129–144. doi:10.1007/978-94-009-4910-2_2. ISBN 978-94-010-8675-2.
- Bain, J. (1980). "Androgen-Progestin Combinations: Clinical Trials". Regulation of Male Fertility: 85–91. doi:10.1007/978-94-009-8875-0_9. ISBN 978-94-009-8877-4.
- Petry, R.; Pfizenmayer, K. (1973). "Möglichkeiten der medikamentösen Fertilitätshemmung beim Mann". Deutsche Medizinische Wochenschrift. 98 (38): 1775–1779. doi:10.1055/s-0028-1107127. ISSN 0012-0472. PMID 4742513.
- Sundsfjord JA, Aakvaag A, Norman N (August 1971). "Reduced plasma testosterone and LH in young men during progesterone administration". J. Reprod. Fertil. 26 (2): 263–5. doi:10.1530/jrf.0.0260263. PMID 5558416.
- Turkmen S, Backstrom T, Wahlstrom G, Andreen L, Johansson IM (2011). "Tolerance to allopregnanolone with focus on the GABA-A receptor". Br. J. Pharmacol. 162 (2): 311–27. doi:10.1111/j.1476-5381.2010.01059.x. PMC 3031054. PMID 20883478.
- Follesa P, Concas A, Porcu P, Sanna E, Serra M, Mostallino MC, Purdy RH, Biggio G (2001). "Role of allopregnanolone in regulation of GABA(A) receptor plasticity during long-term exposure to and withdrawal from progesterone". Brain Res. Brain Res. Rev. 37 (1–3): 81–90. doi:10.1016/s0165-0173(01)00125-4. PMID 11744076.
- Schiller CE, Schmidt PJ, Rubinow DR (2014). "Allopregnanolone as a mediator of affective switching in reproductive mood disorders". Psychopharmacology. 231 (17): 3557–67. doi:10.1007/s00213-014-3599-x. PMC 4135022. PMID 24846476.
- Bäckström T, Haage D, Löfgren M, Johansson IM, Strömberg J, Nyberg S, Andréen L, Ossewaarde L, van Wingen GA, Turkmen S, Bengtsson SK (2011). "Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons". Neuroscience. 191: 46–54. doi:10.1016/j.neuroscience.2011.03.061. PMID 21600269.
- Andréen L, Sundström-Poromaa I, Bixo M, Andersson A, Nyberg S, Bäckström T (February 2005). "Relationship between allopregnanolone and negative mood in postmenopausal women taking sequential hormone replacement therapy with vaginal progesterone". Psychoneuroendocrinology. 30 (2): 212–24. doi:10.1016/j.psyneuen.2004.07.003. PMID 15471618.
Further reading
- Sitruk-Ware R, Bricaire C, De Lignieres B, Yaneva H, Mauvais-Jarvis P (October 1987). "Oral micronized progesterone. Bioavailability pharmacokinetics, pharmacological and therapeutic implications--a review". Contraception. 36 (4): 373–402. doi:10.1016/0010-7824(87)90088-6. PMID 3327648.
- Simon JA (December 1995). "Micronized progesterone: vaginal and oral uses". Clinical Obstetrics and Gynecology. 38 (4): 902–14. doi:10.1097/00003081-199538040-00024. PMID 8616985.
- Ruan X, Mueck AO (November 2014). "Systemic progesterone therapy--oral, vaginal, injections and even transdermal?". Maturitas. 79 (3): 248–55. doi:10.1016/j.maturitas.2014.07.009. PMID 25113944.