Steroidogenesis inhibitor
A steroidogenesis inhibitor, also known as a steroid biosynthesis inhibitor, is a type of drug which inhibits one or more of the enzymes that are involved in the process of steroidogenesis, the biosynthesis of endogenous steroids and steroid hormones.[1] They may inhibit the production of cholesterol and other sterols, sex steroids such as androgens, estrogens, and progestogens, corticosteroids such as glucocorticoids and mineralocorticoids, and neurosteroids.[1][2] They are used in the treatment of a variety of medical conditions that depend on endogenous steroids.[1]
| Steroidogenesis inhibitor | |
|---|---|
| Drug class | |
| Class identifiers | |
| Synonyms | Steroid biosynthesis inhibitor; Steroid synthesis inhibitor |
| Use | Various |
| Biological target | Steroidogenic enzymes |
| Chemical class | Steroidal; Nonsteroidal |
| In Wikidata | |
Steroidogenesis inhibitors are analogous in effect and use to antigonadotropins (which specifically inhibit sex steroid production), but work via a different mechanism of action; whereas antigonadotropins suppress gonadal production of sex steroids by effecting negative feedback on and thereby suppressing the hypothalamic-pituitary-gonadal axis, steroidogenesis inhibitors directly inhibit the enzymatic biosynthesis of steroids.[1]
Types, examples, and uses
Cholesterol synthesis inhibitors
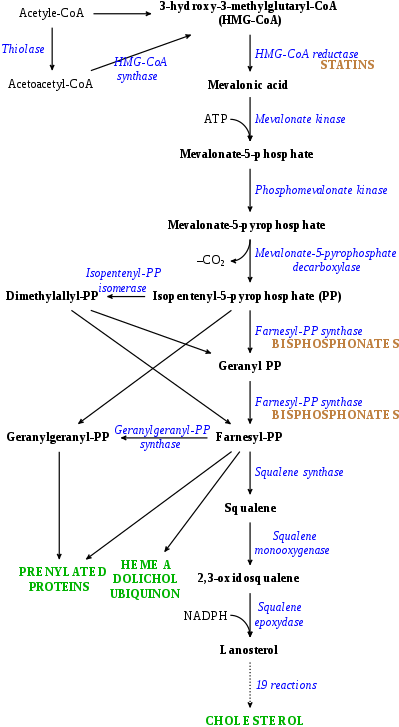
Acetyl-CoA to lanosterol inhibitors
- HMG-CoA reductase (HMGCR) inhibitors, also known as statins, prevent the conversion of HMG-CoA into mevalonic acid, a relatively early step in the biosynthesis of cholesterol from acetyl coenzyme A (acetyl-CoA), and thereby decrease cholesterol levels.[3] Examples of statins include atorvastatin, lovastatin, rosuvastatin, and simvastatin.[3] They are used in the treatment of hypercholesterolemia for the purpose of lowering the risk of atherosclerosis-related cardiovascular disease.[3]
- Farnesyl pyrophosphate synthase (FPPS) inhibitors prevent the conversion of isopentenyl pyrophosphate (IPP) into farnesyl pyrophosphate (FPP), a mid-range step in the biosynthesis of cholesterol from acetyl-CoA, and thereby inhibit cholesterol production.[4][5] They notably do not significantly lower circulating levels of cholesterol however, and hence, unlike statins, are not suitable for the treatment of hypercholesterolemia.[6] The main examples of FPPS inhibitors are nitrogenous bisphosphonates such as alendronate, ibandronate, pamidronate, risedronate, and zoledronate, which are used in the treatment of osteoporosis.[4][5]
- Other early-stage cholesterol synthesis inhibitors like colestolone.[7][8][9]
Lanosterol to cholesterol inhibitors
- 7-Dehydrocholesterol reductase (7-DHCR) inhibitors such as AY-9944 and BM-15766 inhibit the production of cholesterol from 7-dehydrocholesterol, one of the last steps in cholesterol biosynthesis.[10][11] Loss-of-function mutations in the gene encoding 7-DHCR result in Smith–Lemli–Opitz syndrome (SLOS) and dramatic accumulation of 7-dehydrocholesterol.[10][11] 7-DHCR inhibitors produce an acquired form of SLOS in animals, and as such, like 24-DHCR inhibitors (see below), are probably too toxic to be used clinically.[10][11]
- 24-Dehydrocholesterol reductase (24-DHCR) inhibitors such as azacosterol and triparanol inhibit the production of cholesterol from desmosterol, one of the last steps in cholesterol biosynthesis, and were formerly used to treat hypercholesterolemia, but were withdrawn from the market due to toxicity caused by accumulation of desmosterol in tissues.[12][13]
Steroid hormone synthesis inhibitors
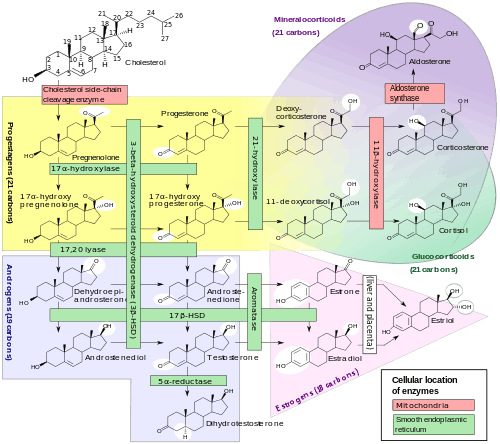
Non-specific steroid hormone synthesis inhibitors
- Cholesterol side-chain cleavage enzyme (P450scc, CYP11A1) inhibitors such as aminoglutethimide,[15] ketoconazole,[16] and mitotane[16] inhibit the production of pregnenolone from cholesterol and thereby prevent the synthesis of all steroid hormones.[17][15] They have been used to inhibit corticosteroid synthesis in the treatment of Cushing's syndrome and adrenocortical carcinoma,[18] and ketoconazole has also been used to inhibit androgen production in the treatment of prostate cancer.[15][19]
- 3β-Hydroxysteroid dehydrogenase (3β-HSD) inhibitors such as amphenone B,[20] azastene, cyanoketone, epostane, mitotane,[16] and trilostane inhibit the conversion of Δ5-3β-hydroxysteroids into Δ4-3-ketosteroids and thereby inhibit the production of most of the steroid hormones.[21] Due to inhibition of progesterone biosynthesis, they have been investigated as contraceptives and abortifacients (though ultimately have never been marketed for this indication),[21] and trilostane was formerly used to inhibit corticosteroid synthesis in the treatment of Cushing's syndrome.[22]
- 17α-Hydroxylase/17,20-lyase (CYP17A1) inhibitors such as abiraterone acetate, etomidate,[16] galeterone, ketoconazole,[16] and orteronel inhibit the production of androgens and glucocorticoids and are used to reduce androgen levels in the treatment of prostate cancer.[17][23] Selective 17,20-lyase inhibitors such as seviteronel inhibit only androgen production without affecting glucocorticoid synthesis and are under development for the treatment of prostate cancer.[24]
Corticosteroid-specific synthesis inhibitors
- 21-Hydroxylase (CYP21A2) inhibitors prevent the production of corticosteroids from progesterone and 17α-hydroxyprogesterone.[17]
- 11β-Hydroxylase (CYP11B1) inhibitors such as amphenone B,[20] etomidate,[16] ketoconazole,[16] metyrapone,[16] mitotane,[16] and osilodrostat[25] inhibit the production of the potent corticosteroids cortisol, corticosterone, and aldosterone from the less potent corticosteroids 11-deoxycorticosterone and 11-deoxycortisol and are used in the diagnosis and treatment of Cushing's syndrome.[17]
- Aldosterone synthase (CYP11B2) inhibitors such as metyrapone,[26] mitotane,[16] and osilodrostat[25] prevent the production of the potent mineralocorticoid aldosterone from the less potent mineralocorticoid corticosterone.[17] Osilodrostat was investigated for the treatment of hypertension, heart failure, and renal disease, but development for these indications was discontinued.[25]
Sex steroid-specific synthesis inhibitors
- 17β-Hydroxysteroid dehydrogenase (17β-HSD) inhibitors prevent the reversible conversion of the weak androgens dehydroepiandrosterone (DHEA) and 4-androstenedione into the more potent androgen testosterone and the weak estrogen estrone into the more potent estrogen estradiol.[27]
- 5α-Reductase inhibitors (5-ARIs) such as finasteride, dutasteride, epristeride, and alfatradiol[28] prevent the conversion of testosterone into the more potent androgen dihydrotestosterone (DHT) and are used in the treatment of benign prostatic hyperplasia (BPH) and androgenic alopecia (pattern hair loss).[29] These drugs also inhibit the formation of neurosteroids such as allopregnanolone, tetrahydrodeoxycorticosterone (THDOC), and 3α-androstanediol from progesterone, 11-deoxycorticosterone, and DHT, respectively, which may contribute to side effects such as depression and sexual dysfunction.[2]
- Aromatase inhibitors (AIs) such as aminoglutethimide, anastrozole, exemestane, letrozole, and testolactone inhibit the production of estrogens from androgens and are used mainly in the treatment of estrogen receptor-positive breast cancer.[30]
- Steroid sulfotransferase (SST) inhibitors prevent the conversion of steroid hormones such as estrone and DHEA into hormonally inactive steroid sulfates.[31] Although hormonally inactive, some steroid sulfates, such as pregnenolone sulfate and DHEA sulfate, are important neurosteroids.[32][33]
- Steroid sulfatase (STS) inhibitors such as estradiol sulfamate, estrone sulfamate, irosustat, and danazol[34] inhibit the conversion of steroid sulfates such as estrone sulfate and DHEA sulfate into their hormonally active forms.[35][36] They have potential applications in the treatment of breast cancer and endometriosis, and are currently under investigation for such indications.[35][36]
Other steroid synthesis inhibitors
- Lanosterol 14α-demethylase (CYP51A1) inhibitors such as clotrimazole, fluconazole, itraconazole, ketoconazole, miconazole, and voriconazole prevent the production of ergosterol from lanosterol.[17][37] Ergosterol is absent in animals but is an essential component of the cell membranes of many fungi and protozoa, and so lanosterol 14α-demethylase inhibitors are used as antifungals and antiprotozoals in the treatment of infections.[37]
List of steroid metabolism modulators
See also
- Steroidogenic enzyme
- Neurosteroidogenesis inhibitor
References
- Vanden Bossche H (1992). "Inhibitors of P450-dependent steroid biosynthesis: from research to medical treatment". J. Steroid Biochem. Mol. Biol. 43 (8): 1003–21. doi:10.1016/0960-0760(92)90328-G. PMID 22217845.
- Tvrdeić, Ante; Poljak, Ljiljana (2016). "Neurosteroids, GABAA receptors and neurosteroid based drugs: are we witnessing the dawn of the new psychiatric drugs?". Endocrine Oncology and Metabolism. 2 (1): 60–71. doi:10.21040/eom/2016.2.7. ISSN 1849-8922.
- Stephen E. Wolverton (18 October 2012). Comprehensive Dermatologic Drug Therapy E-Book. Elsevier Health Sciences. pp. 415–. ISBN 1-4557-3801-8.
- Frank J. Dowd; Bart Johnson; Angelo Mariotti (3 September 2016). Pharmacology and Therapeutics for Dentistry - E-Book. Elsevier Health Sciences. pp. 426–. ISBN 978-0-323-44595-5.
- Nuclear Receptors in Development and Disease. Elsevier Science. 17 May 2017. pp. 88–. ISBN 978-0-12-802196-5.
- Francesco Clementi; Guido Fumagalli (9 February 2015). General and Molecular Pharmacology: Principles of Drug Action. John Wiley & Sons. pp. 442–. ISBN 978-1-118-76857-0.
- https://www.google.com/patents/US5112815
- Schroepfer GJ, Chu AJ, Needleman DH, Izumi A, Nguyen PT, Wang KS, Little JM, Sherrill BC, Kisic A (1988). "Inhibitors of sterol synthesis. Metabolism of 5 alpha-cholest-8(14)-en-3 beta-ol-15-one after intravenous administration to bile duct-cannulated rats". J. Biol. Chem. 263 (9): 4110–23. PMID 3346239.
- Schroepfer GJ, Parish EJ, Kisic A, Jackson EM, Farley CM, Mott GE (1982). "5 alpha-Cholest-8(14)-en-3 beta-ol-15-one, a potent inhibitor of sterol biosynthesis, lowers serum cholesterol and alters distributions of cholesterol in lipoproteins in baboons". Proc. Natl. Acad. Sci. U.S.A. 79 (9): 3042–6. doi:10.1073/pnas.79.9.3042. PMC 346345. PMID 6953447.
- E. Gilbert-Barness; L.A. Barness; P.M. Farrell (6 January 2017). Metabolic Diseases: Foundations of Clinical Management, Genetics, and Pathology. IOS Press. pp. 336–337. ISBN 978-1-61499-718-4.
- Robert Bittman (11 November 2013). Cholesterol: Its Functions and Metabolism in Biology and Medicine. Springer Science & Business Media. pp. 130–. ISBN 978-1-4615-5901-6.
- Peter M. Elias (21 January 2016). Advances in Lipid Research: Skin Lipids. Elsevier. pp. 218–. ISBN 978-1-4832-1545-7.
- Carl A. Burtis; Edward R. Ashwood; David E. Bruns (14 October 2012). Tietz Textbook of Clinical Chemistry and Molecular Diagnostics - E-Book. Elsevier Health Sciences. pp. 733–. ISBN 1-4557-5942-2.
- Häggström M, Richfield D (2014). "Diagram of the pathways of human steroidogenesis". WikiJournal of Medicine. 1 (1). doi:10.15347/wjm/2014.005. ISSN 2002-4436.
- Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 735–. ISBN 978-0-7817-1750-2.
- J. Larry Jameson; Leslie J. De Groot (18 May 2010). Endocrinology - E-Book: Adult and Pediatric. Elsevier Health Sciences. pp. 301–302. ISBN 1-4557-1126-8.
- Paul R. Ortiz de Montellano (13 March 2015). Cytochrome P450: Structure, Mechanism, and Biochemistry. Springer. pp. 851–879. ISBN 978-3-319-12108-6.
- Dimitrios A. Linos; Jon A. van Heerden (5 December 2005). Adrenal Glands: Diagnostic Aspects and Surgical Therapy. Springer Science & Business Media. pp. 171–. ISBN 978-3-540-26861-1.
- Waun Ki Hong; American Association for Cancer Research (2010). Holland-Frei Cancer Medicine 8. PMPH-USA. pp. 756–. ISBN 978-1-60795-014-1.
- L Martini (2 December 2012). Hormonal Steroids Biochemistry, Pharmacology, and Therapeutics: Proceedings of the First International Congress on Hormonal Steroids. Elsevier. pp. 383–. ISBN 978-0-323-14465-0.
- Ray S, Sharma I (1987). "Development of progesterone antagonists as fertility regulating agents". Pharmazie. 42 (10): 656–61. PMID 3325988.
- Marcello D. Bronstein (1 October 2010). Cushing's Syndrome: Pathophysiology, Diagnosis and Treatment. Springer Science & Business Media. pp. 157–. ISBN 978-1-60327-449-4.
- William D. Figg; Cindy H. Chau; Eric J. Small (14 September 2010). Drug Management of Prostate Cancer. Springer Science & Business Media. pp. 96–98. ISBN 978-1-60327-829-4.
- Stephen Neidle (30 September 2013). Cancer Drug Design and Discovery. Academic Press. pp. 341–. ISBN 978-0-12-397228-6.
- Fleseriu M, Castinetti F (2016). "Updates on the role of adrenal steroidogenesis inhibitors in Cushing's syndrome: a focus on novel therapies". Pituitary. 19 (6): 643–653. doi:10.1007/s11102-016-0742-1. PMC 5080363. PMID 27600150.
- Jürg Müller (6 December 2012). Regulation of Aldosterone Biosynthesis. Springer Science & Business Media. pp. 39–. ISBN 978-3-642-96062-8.
- Jerome F. Strauss; Robert L. Barbieri (28 August 2013). Yen & Jaffe's Reproductive Endocrinology E-Book: Physiology, Pathophysiology, and Clinical Management. Elsevier Health Sciences. pp. 81–82. ISBN 978-1-4557-5972-9.
- Ralph M. Trüeb (26 February 2013). Female Alopecia: Guide to Successful Management. Springer Science & Business Media. pp. 79–. ISBN 978-3-642-35503-5.
- Rob Bradbury (30 January 2007). Cancer. Springer Science & Business Media. pp. 46–50. ISBN 978-3-540-33120-9.
- Aman U. Buzdar (8 November 2007). Endocrine Therapies in Breast Cancer. OUP Oxford. pp. 37–40. ISBN 978-0-19-921814-1.
- Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA (2015). "The Regulation of Steroid Action by Sulfation and Desulfation". Endocr. Rev. 36 (5): 526–63. doi:10.1210/er.2015-1036. PMC 4591525. PMID 26213785.
- Gibbs TT, Russek SJ, Farb DH (2006). "Sulfated steroids as endogenous neuromodulators". Pharmacol. Biochem. Behav. 84 (4): 555–67. doi:10.1016/j.pbb.2006.07.031. PMID 17023038.
- Prough RA, Clark BJ, Klinge CM (2016). "Novel mechanisms for DHEA action". J. Mol. Endocrinol. 56 (3): R139–55. doi:10.1530/JME-16-0013. PMID 26908835.
- Carlström K, Döberl A, Pousette A, Rannevik G, Wilking N (1984). "Inhibition of steroid sulfatase activity by danazol". Acta Obstet Gynecol Scand Suppl. 123: 107–11. doi:10.3109/00016348409156994. PMID 6238495.
- Sadozai H (2013). "Steroid sulfatase inhibitors: promising new therapy for breast cancer". J Pak Med Assoc. 63 (4): 509–15. PMID 23905452.
- Thomas MP, Potter BV (2015). "Estrogen O-sulfamates and their analogues: Clinical steroid sulfatase inhibitors with broad potential". J. Steroid Biochem. Mol. Biol. 153: 160–9. doi:10.1016/j.jsbmb.2015.03.012. PMID 25843211.
- Tekoa L. King; Mary C. Brucker (25 October 2010). Pharmacology for Women's Health. Jones & Bartlett Publishers. pp. 292–. ISBN 978-1-4496-5800-7.
External links
