Dienogest
Dienogest, sold under the brand names Natazia and Qlaira among others, is a progestin medication which is used in birth control pills and in the treatment of endometriosis.[6][7][1][8][9][10] It is also used in menopausal hormone therapy and to treat heavy periods.[8][11][12] Dienogest is available both alone and in combination with estrogens.[13][11] It is taken by mouth.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Alone: Dinagest, Visanne With EV: Natazia, Qlaira With EE: Valette |
| Other names | DNG; Dienogestril; Cyanomethyldienolone; BAY 86-5258; Endometrion; M-18575; MJR-35; SH-660; SH-T00660AA; STS-557; ZK-37659; δ9-17α-Cyanomethyl-19-nortestosterone; 17α-Cyanomethylestra-4,9(10)-dien-17β-ol-3-one; 17β-Hydroxy-3-oxo-19-nor-17α-pregna-4,9-diene-21-nitrile |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | By mouth[1][2] |
| Drug class | Progestogen; Progestin; Steroidal antiandrogen |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 90%[3] |
| Protein binding | Albumin: 90%[3] Free: 10%[3] |
| Metabolism | Liver (reduction, hydroxylation via CYP3A4, removal of cyanomethyl group, conjugation)[3][4] |
| Metabolites | • 9α,10β-Dihydro-DNG[1] • 3,5α-Tetrahydro-DNG[1] (Both said to be inactive)[2][3] |
| Elimination half-life | 7.5–10.7 hours[2] |
| Excretion | Urine[5] |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.167.087 |
| Chemical and physical data | |
| Formula | C20H25NO2 |
| Molar mass | 311.425 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.2 g/cm3 |
| Melting point | 210 to 218 °C (410 to 424 °F) (experimental) |
| Boiling point | 549 °C (1,020 °F) |
SMILES
| |
InChI
| |
| (verify) | |
Side effects of dienogest include menstrual irregularities, headaches, nausea, breast tenderness, depression, and acne, among others.[14] Dienogest is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[1][2] It is a unique progestogen, with strong effects in the uterus.[2] The medication has some antiandrogenic activity, which may help to improve androgen-dependent symptoms like acne, and has no other important hormonal activity.[1][2][7][15][16]
Dienogest was discovered in 1979 and was introduced for medical use in 1995.[17][18][19] Additional formulations of dienogest were approved between 2007 and 2010.[10][20] It is sometimes referred to as a "fourth-generation" progestin.[21][22] Dienogest is marketed widely throughout the world.[13] It is available as a generic medication.[23]
Medical uses
Birth control
Dienogest is used primarily in birth control pills in combination with ethinylestradiol under the brand name Valette.[24][7][25] It is also available in a quadriphasic birth control pill combined with estradiol valerate, marketed as Natazia in the United States and Qlaira in some European countries and Russia.[26][27][28]
Endometriosis
Dienogest is approved as a standalone medication under the brand names Visanne and Dinagest in various places such as Europe, Australia, Japan, Singapore, and Malaysia for the treatment of endometriosis.[29][10][30] It has been found to be equally effective as gonadotropin-releasing hormone agonists (GnRH agonists), such as leuprorelin, in the treatment of endometriosis.[29]
Heavy periods
Birth control pills containing dienogest and estradiol valerate are approved in the United States for the treatment of menorrhagia (heavy menstrual bleeding).[12]
Menopausal symptoms
Dienogest is used in combination with estradiol valerate in the treatment of menopausal symptoms in certain countries such as Germany and the Netherlands.[8][11]
Available forms
Dienogest is available both alone and in combination with estrogens.[13][11] The following formulations are available:[13][11]
- Dienogest 1 mg oral tablets (Dinagest) and 2 mg oral tablets (Valette) – indicated for endometriosis
- Dienogest 2 mg and estradiol valerate 3 mg oral tablets (Natazia) – indicated for contraception and menorrhagia[12]
- Dienogest 2 to 3 mg and estradiol valerate 1 to 3 mg oral tablets (Qlaira) – indicated for contraception
- Dienogest 2 mg and ethinylestradiol 30 µg oral tablets (Valette) – indicated for contraception
- Dienogest 2 mg and estradiol valerate 1 or 2 mg oral tablets (various) – indicated for menopausal hormone therapy
The availability of these formulations differs by country (see Availability).[13]
Contraindications
Contraindications of dienogest include active venous thromboembolism, previous or current cardiovascular disease, diabetes with cardiovascular complications, previous or current severe liver disease or tumors, hormone-dependent cancers such as breast cancer, and undiagnosed vaginal bleeding.[10][31]
Side effects
Side effects associated with dienogest are the same as those expected of a progestogen.[7] They include menstrual irregularities, headaches, nausea, breast tenderness, depression, acne, weight gain, flatulence, and others.[14] Dienogest produces no androgenic side effects and has little effect on metabolic and lipid hemostatic parameters.[32]
Birth control pills containing estradiol valerate/dienogest are associated with a significantly increased risk of venous thromboembolism.[33] However, they are associated with a significantly lower risk of venous thromboembolism than birth control pills containing ethinylestradiol and a progestin.[33]
Overdose
In safety studies, dienogest has been assessed in women with endometriosis at high doses of as much as 20 mg/day for up to 24 weeks and produced no clinically relevant effects on lipid metabolism, liver enzymes, the coagulatory system, or thyroid metabolism.[11]
Interactions
Dienogest is metabolized mainly by the cytochrome P450 enzyme CYP3A4,[3][10] and for this reason, inhibitors and inducers of CYP3A4 can alter the amount of exposure to dienogest when administered concomitantly with it.[10] (For a list of CYP3A4 inhibitors and inducers, see here.) The strong CYP3A4 inhibitors ketoconazole and erythromycin have been found to increase exposure to dienogest by up to 3-fold, whereas the strong CYP3A4 inducer rifampicin (rifampin) was found to decrease steady-state and area-under-curve concentrations of dienogest by 50% and 80%, respectively.[10]
Pharmacology
Pharmacodynamics
Dienogest has progestogenic activity, possibly some antiprogestogenic activity, and has antiandrogenic activity.[2][7][1][3] The medication does not interact with the estrogen receptor, the glucocorticoid receptor, or the mineralocorticoid receptor, and hence has no estrogenic, glucocorticoid, or antimineralocorticoid activity.[2][7][1][3] Because of its relatively high selectivity as a progestogen, dienogest may have favorable safety and tolerability compared to various other progestins.[1][2]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG | |
|---|---|---|---|---|---|---|---|---|
| Dienogest | 5 | 10 | 0 | 1 | 0 | 0 | 0 | |
| 9α,10β-Dihydrodienogest | 26 | 13 | ? | ? | ? | ? | ? | |
| 3α,5α-Tetrahydrodienogest | 19 | 16 | ? | ? | ? | ? | ? | |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. Sources: [1] | ||||||||
Progestogenic activity
Dienogest is an agonist of the progesterone receptor (PR), and hence is a progestogen.[2][7][1] It has relatively weak affinity for the PR in vitro in human uterine tissue, about 10% that of progesterone.[2][7][3] Despite its low affinity for the PR however, dienogest has high progestogenic activity in vivo.[2] In addition, although its metabolites, such as 9α,10β-dihydrodienogest and 3α,5α-tetrahydrodienogest, have greater affinity for the PR than does dienogest itself, the medication is not considered to be a prodrug.[2][1][7][3]
Dienogest has been described as "special" progestogen, possessing low or moderate antigonadotropic efficacy but strong or very strong endometrial efficacy.[2][7] In relation to its endometrial activity, dienogest is said to be one of the strongest progestogens available.[2] The high endometrial activity of dienogest underlies its ability to stabilize the menstrual cycle when combined with either ethinylestradiol or estradiol valerate (which has lower relative effects on the uterus compared to ethinylestradiol) in birth control pills, and also its use in the treatment of endometriosis.[2] The combination of most other progestins with estradiol or an estradiol ester like estradiol valerate as birth control pills was unsatisfactory due to a high incidence of irregular menstrual bleeding.[2] This is a property that ethinylestradiol does not share with estradiol, because of its resistance to metabolism in the endometrium and hence its greater relative effects in this part of the body.[1] In contrast to other progestins, due to its high endometrial efficacy, the combination of dienogest with estradiol valerate in birth control pills is able to prevent breakthrough bleeding, and is uniquely able to treat heavy menstrual bleeding.[2] The absence of withdrawal bleeding, otherwise known as "silent menstruation", also may occur.[2]
Unlike other progestogens, except in the case of its strong effects in the uterus, dienogest has been described as lacking antiestrogenic effects, and does not antagonize beneficial effects of estradiol, for instance in the metabolic and vascular systems.[2]
Dienogest showed some possible antiprogestogenic activity in one animal bioassay when administered before but not at the same time as progesterone.[7]
The minimum effective dose of oral dienogest required to inhibit ovulation is 1 mg/day.[2][1] The inhibition of ovulation by dienogest occurs mainly via a direct peripheral action in the ovary of inhibiting folliculogenesis as opposed to a central action of inhibiting gonadotropin secretion.[2][1] Oral therapy with 2 mg/day dienogest in cyclical premenopausal women reduced serum progesterone levels to anovulatory levels, but circulating levels of luteinizing hormone and follicle-stimulating hormone were not considerably affected.[2] At this dosage, estradiol levels are reduced to early follicular phase levels of about 30 to 50 pg/mL.[2] Such levels are insufficient for reactivation of endometrioses, but are sufficient to avoid menopausal-like symptoms such as hot flashes and bone loss.[2] This is in contrast to gonadotropin-releasing hormone analogues (GnRH analogues), which suppress estradiol levels to lower concentrations and readily induce menopausal-like symptoms.[2]
Dienogest appears to have similar effects in the breasts as norethisterone acetate, and may likewise increase the risk of breast cancer when combined with an estrogen in postmenopausal women, although this has yet to be confirmed in clinical studies.[2]
Antigonadotropic effects
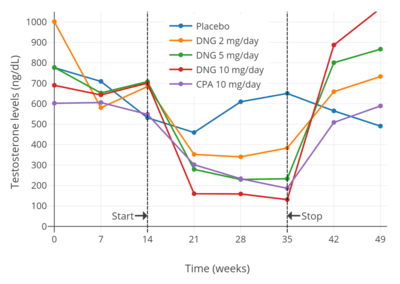
Dienogest has been found to suppress testosterone levels in men by 43% at 2 mg/day, 70% at 5 mg/day, and 81% at 10 mg/day.[34][35] The suppression of testosterone levels with 10 mg/day dienogest was comparable to that with 10 mg/day cyproterone acetate.[35][34] In general, progestogens are able to suppress testosterone levels in men by a maximum of about 70 to 80% at sufficiently high doses.[36][37][38][39][40]
Antiandrogenic activity
Dienogest is one of the only 19-nortestosterone derivative progestins that does not have androgenic properties.[1][2] In fact, it is actually an antagonist of the androgen receptor (AR), and hence has antiandrogenic activity.[2][3][1] The antiandrogenic activity of dienogest in the Hershberger test is about 30 to 40% of that of cyproterone acetate.[2][3][1] It may be able to improve androgen-dependent symptoms such as acne and hirsutism.[7][15][2] Metabolites of dienogest, such as 9α,10β-dihydrodienogest and 3α,5α-tetrahydrodienogest, show greater affinity for the AR than does dienogest itself.[1] Dienogest has no affinity for sex hormone-binding globulin (SHBG), and hence does not displace testosterone or estradiol from this plasma protein or increase the free fractions of these hormones.[2]
Other activities
Dienogest does not inhibit or induce CYP3A4, unlike many other related progestins.[7][1] Because of this, dienogest may have a lower propensity for drug interactions.[2]
Pharmacokinetics
Dienogest is rapidly absorbed with oral administration and has high bioavailability of approximately 90%.[3] Peak levels of dienogest occur within approximately 2 hours after an oral dose.[7] The pharmacokinetics of dienogest are linear; single oral doses of dienogest were found to result in maximal levels of 28 ng/mL with 1 mg, 54 ng/mL with 2 mg, 101 ng/mL with 4 mg, and 212 ng/mL with 8 mg.[7] The corresponding area-under-the-curve levels were 306, 577, 1153, and 2293 ng/mL, respectively.[7] Dienogest reaches steady-state concentrations within 6 days of continuous administration, and does not accumulate in the body.[7][3] The plasma protein binding of dienogest is 90%, with a relatively high free fraction of 10%.[7] It is exclusively bound to albumin, with no binding to SHBG or corticosteroid-binding globulin.[2][7][3] The lack of affinity of dienogest for SHBG is in contrast to most other 19-nortestosterone progestins.[2] The volume of distribution of dienogest is relatively low at 40 L.[7]
Dienogest is metabolized in the liver.[2][3] Metabolic pathways of dienogest include reduction of its Δ4-3-keto group, hydroxylation mainly via CYP3A4, removal of its C17α cyanomethyl group, and conjugation.[2][3] The metabolites of dienogest are quickly excreted and are said to be mostly inactive.[2] The elimination half-life of dienogest is relatively short at approximately 7.5 to 10.7 hours.[2][7][3][4] The short half-life of dienogest relative to other 19-nortestosterone progestins is in part due to its lack of binding to SHBG and hence prolongation in the circulation.[2] The clearance of dienogest is 3 L/h.[7] It is eliminated mainly in the urine, both as sulfate and glucuronide conjugates and as free steroid.[5]
Chemistry
Dienogest, also known as δ9-17α-cyanomethyl-19-nortestosterone or as 17α-cyanomethylestra-4,9-dien-17β-ol-3-one, is a synthetic estrane steroid and a derivative of testosterone.[41][7][42] It is a member of the estrane subgroup of the 19-nortestosterone family of progestins, but unlike most other 19-nortestosterone progestins, is not a derivative of norethisterone (17α-ethynyl-19-nortestosterone).[22][43][44] This is because it uniquely possesses a cyanomethyl group at the C17α position rather than the usual ethynyl group.[42][22] It is also unique among most 19-nortestosterone progestins in that it has a double bond between the C9 and C10 positions.[42] Dienogest is the C17α cyanomethyl derivative of the anabolic–androgenic steroid (AAS) dienolone, as well as the C17α cyanomethyl analogue of the AAS methyldienolone (17α-methyldienolone) and ethyldienolone (17α-ethyldienolone).[41]
In terms of structure–activity relationships, the C17α cyanomethyl group of dienogest is responsible for its unique antiandrogenic instead of androgenic activity relative to other 19-nortestosterone progestins.[42] A loss of ability to activate the AR is also seen with other testosterone derivatives with extended-length C17α substitutions such as topterone (propyltestosterone) (compare to the AAS ethyltestosterone and methyltestosterone) and allylestrenol (compare to the AAS ethylestrenol).[45][46] Studies with steroids similar to dienogest (e.g., dienolone) have found that the introduction of a double bond between the C9 and C10 positions is associated with similar/almost unchanged affinity for the PR and AR.[47] On the other hand, the C9(10) double bond of dienogest appears to inhibit metabolism via 5α-reductase and/or 5β-reductase, which is the major metabolic route for other 19-nortestosterone progestins like norethisterone, norgestrel, and etonogestrel, and this may serve to improve the metabolic stability and potency of dienogest.[48][49]
History
Dienogest was synthesized in 1979 in Jena, Germany under the leadership of Kurt Ponsold, was initially referred to as STS-557.[17][18] It was found that its potency was 10 times that of levonorgestrel.[50] The first product on the market to contain dienogest was a combined birth control pill (with ethinylestradiol), Valette, introduced in 1995 and made by Jenapharm.[19] In 2007, dienogest was introduced as Dinagest in Japan for the treatment of endometriosis, and it was subsequently marketed for this indication as Visanne in Europe and Australia in December 2009 and April 2010, respectively.[10] Qlaira was introduced in Europe in 2009 and Natazia was introduced in the United States in 2010.[20]
Society and culture
Generic names
Dienogest is the generic name of the drug and its INN, USAN, BAN, and JAN, while diénogest is its DCF.[41][13] It is also known by its synonyms dienogestril and cyanomethyldienolone as well as by its numerous former developmental code names including BAY 86-5258, M-18575, MJR-35, SH-660, SH-T00660AA, STS-557, and ZK-37659.[41][13]
Brand names
Dienogest is marketed in combination with estradiol valerate as a birth control pill primarily under the brand names Natazia and Qlaira and in combination with ethinylestradiol as a birth control pill primarily under the brand name Valette, although these combinations are marketed under numerous other brand names as well.[13] In the case of the dienogest and estradiol valerate birth control pill, these other brand names include Gianda and Klaira.[13] Dienogest is also marketed in combination with estradiol valerate for use in menopausal hormone therapy under a variety of brand names including Climodien, Climodiène, Estradiol Valeraat / Dienogest, Klimodien, lafamme, Lafleur, Mevaren, Valerix, and Velbienne.[13] Dienogest is marketed as a standalone medication for the treatment of endometriosis primarily under the brand name Visanne, but is also available under the brand names Alondra, Dinagest, Disven, Visabelle, and Visannette in various countries.[13]
Availability
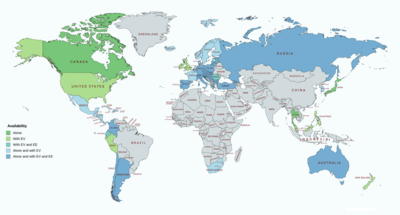
Dienogest is available both alone and in combination with ethinylestradiol and estradiol valerate widely throughout the world, including but not limited to Canada, Europe, Latin America, and Southeast Asia.[13][51][52] It is available specifically as a standalone medication in Canada, Europe, Latin America, Russia, Australia, South Africa, Georgia, Israel, Japan, South Korea, Hong Kong, and Thailand.[13][51][52] It is notably not available as a standalone medication in the United States or the United Kingdom.[13][51][52]
Research
Dienogest has been studied as a form of male hormonal contraception.[53][34][54] As of July 2018, dienogest is in phase III clinical trials in Japan for the treatment of adenomyosis and dysmenorrhea.[8] The combination of estradiol valerate and dienogest is in pre-registration in Europe for the treatment of acne.[55] Dienogest is also being evaluated for the potential treatment of anorexia nervosa.[56]
References
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Ruan X, Seeger H, Mueck AO (April 2012). "The pharmacology of dienogest". Maturitas. 71 (4): 337–44. doi:10.1016/j.maturitas.2012.01.018. PMID 22364708.
- Bińkowska, Małgorzata; Woroń, Jarosław (2015). "Progestogens in menopausal hormone therapy". Menopausal Review. 14 (2): 134–143. doi:10.5114/pm.2015.52154. ISSN 1643-8876. PMC 4498031. PMID 26327902.
- Stanczyk FZ (2003). "All progestins are not created equal". Steroids. 68 (10–13): 879–90. doi:10.1016/j.steroids.2003.08.003. PMID 14667980.
- Bizzarri N, Remorgida V, Leone Roberti Maggiore U, Scala C, Tafi E, Ghirardi V, Salvatore S, Candiani M, Venturini PL, Ferrero S (2014). "Dienogest in the treatment of endometriosis". Expert Opin Pharmacother. 15 (13): 1889–902. doi:10.1517/14656566.2014.943734. PMID 25069386.
- https://www.bayer.ca/static/documents/news/en/VISANNE-PM-EN-19JUN2015-182736.pdf
- Foster RH, Wilde MI (1998). "Dienogest". Drugs. 56 (5): 825–33, discussion 834–5. doi:10.2165/00003495-199856050-00007. PMID 9829156.
- "Dienogest - Bayer HealthCare Pharmaceuticals/Mochida Pharmaceutical - AdisInsight".
- Tommaso Falcone; William W. Hurd (22 May 2013). Clinical Reproductive Medicine and Surgery: A Practical Guide. Springer Science & Business Media. pp. 300–. ISBN 978-1-4614-6837-0.
Dienogest is a 19-nortestosterone derivative that is approved in the European Union for the treatment of endometriosis. It is not available in the United States as a separate drug. It is only available in the oral contraceptive Natazia (Bayer Pharmaceuticals, Montville, NJ, USA) (estradiol valerate/dienogest), which is a newer four-phasic pack that contains dienogest.
- McCormack PL (2010). "Dienogest: a review of its use in the treatment of endometriosis". Drugs. 70 (16): 2073–88. doi:10.2165/11206320-000000000-00000. PMID 20964453.
- Bartsch, V., & Römer, T. (2015). Gynaecological uses of dienogest alone and in combination with oestrogens. http://www.jmdrev.com/fileadmin/user_upload/Medien-Dateien/JMDR_Dienogest_E_2015.pdf
- Micks EA, Jensen JT (2013). "Treatment of heavy menstrual bleeding with the estradiol valerate and dienogest oral contraceptive pill". Adv Ther. 30 (1): 1–13. doi:10.1007/s12325-012-0071-3. PMID 23239397.
- "Dienogest".
- "Dienogest". Aust Prescr. 38 (4): 138–9. 2015. doi:10.18773/austprescr.2015.050. PMC 4653971. PMID 26648643.
- Raudrant D, Rabe T (2003). "Progestogens with antiandrogenic properties". Drugs. 63 (5): 463–92. doi:10.2165/00003495-200363050-00003. PMID 12600226.
- Regidor PA, Schindler AE (2017). "Antiandrogenic and antimineralocorticoid health benefits of COC containing newer progestogens: dienogest and drospirenone". Oncotarget. 8 (47): 83334–83342. doi:10.18632/oncotarget.19833. PMC 5669973. PMID 29137347.
- Menzenbach B; Hübner M; K. Ponsold (1984). "Untersuchungen zur Bromierung/Dehydrobromierung von 17-Cyanmethyl-17-hydroxy-östr-5(10)-en-3-on". Journal für Praktische Chemie. 326 (6): 893–898. doi:10.1002/prac.19843260606.
- Kaufmann G, Dautzenberg H, Henkel H, et al. (August 1999). "Nitrile hydratase from Rhodococcus erythropolis: metabolization of steroidal compounds with a nitrile group". Steroids. 64 (8): 535–40. doi:10.1016/S0039-128X(99)00028-8. PMID 10493599.
- "Archived copy". Archived from the original on 2008-03-07. Retrieved 2008-01-18.CS1 maint: archived copy as title (link)
- Guida M, Bifulco G, Di Spiezio Sardo A, Scala M, Fernandez LM, Nappi C (2010). "Review of the safety, efficacy and patient acceptability of the combined dienogest/estradiol valerate contraceptive pill". International Journal of Women's Health. 2: 279–90. doi:10.2147/IJWH.S6954. PMC 2990895. PMID 21151673.
- Paula Briggs; Gabor Kovacs (11 July 2013). Contraception: A Casebook from Menarche to Menopause. Cambridge University Press. pp. 52–. ISBN 978-1-107-43611-4.
- Howard J.A. Carp (9 April 2015). Progestogens in Obstetrics and Gynecology. Springer. pp. 112–113, 170–. ISBN 978-3-319-14385-9.
- "Generic Natazia Availability".
- Pérez-Campos EF (April 2010). "Ethinylestradiol/dienogest in oral contraception". Drugs. 70 (6): 681–9. doi:10.2165/11536320-000000000-00000. PMID 20394455.
- Wiegratz I, Mittmann K, Dietrich H, Zimmermann T, Kuhl H (2006). "Fertility after discontinuation of treatment with an oral contraceptive containing 30 microg of ethinyl estradiol and 2 mg of dienogest". Fertil. Steril. 85 (6): 1812–9. doi:10.1016/j.fertnstert.2005.11.052. PMID 16759929.
- Hoy SM, Scott LJ (August 2009). "Estradiol valerate/dienogest: in oral contraception". Drugs. 69 (12): 1635–46. doi:10.2165/11202820-000000000-00000. PMID 19678714.
- Whalen KL, Rose R (October 2011). "Estradiol valerate/dienogest: a novel oral contraceptive". Ann Pharmacother. 45 (10): 1256–61. doi:10.1345/aph.1Q216. PMID 21917554.
- Fruzzetti F, Trémollieres F, Bitzer J (May 2012). "An overview of the development of combined oral contraceptives containing estradiol: focus on estradiol valerate/dienogest". Gynecol. Endocrinol. 28 (5): 400–8. doi:10.3109/09513590.2012.662547. PMC 3399636. PMID 22468839.
- Andres Mde P, Lopes LA, Baracat EC, Podgaec S (September 2015). "Dienogest in the treatment of endometriosis: systematic review". Arch. Gynecol. Obstet. 292 (3): 523–9. doi:10.1007/s00404-015-3681-6. PMID 25749349.
- "Dienogest for the treatment of endometriosis" (PDF). London New Drugs Group. Archived from the original (PDF) on 2011-10-02. Retrieved 2010-12-07.
- http://db.cbg-meb.nl/IB-teksten/h104058.pdf
- Wiegratz I, Lee JH, Kutschera E, Bauer HH, von Hayn C, Moore C, Mellinger U, Winkler UH, Gross W, Kuhl H (2002). "Effect of dienogest-containing oral contraceptives on lipid metabolism". Contraception. 65 (3): 223–9. doi:10.1016/S0010-7824(01)00310-9. PMID 11929644.
- Fruzzetti F, Cagnacci A (2018). "Venous thrombosis and hormonal contraception: what's new with estradiol-based hormonal contraceptives?". Open Access J Contracept. 9: 75–79. doi:10.2147/OAJC.S179673. PMC 6239102. PMID 30519125.
- Meriggiola MC, Bremner WJ, Costantino A, Bertaccini A, Morselli-Labate AM, Huebler D, Kaufmann G, Oettel M, Flamigni C (May 2002). "Twenty-one day administration of dienogest reversibly suppresses gonadotropins and testosterone in normal men". J. Clin. Endocrinol. Metab. 87 (5): 2107–13. doi:10.1210/jcem.87.5.8514. hdl:1773/4465. PMID 11994349.
- Nieschlag E, Zitzmann M, Kamischke A (November 2003). "Use of progestins in male contraception". Steroids. 68 (10–13): 965–72. doi:10.1016/S0039-128X(03)00135-1. PMID 14667989.
- Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (25 August 2011). Campbell-Walsh Urology: Expert Consult Premium Edition: Enhanced Online Features and Print, 4-Volume Set. Elsevier Health Sciences. pp. 2938–. ISBN 978-1-4160-6911-9.
- Knuth UA, Hano R, Nieschlag E (1984). "Effect of flutamide or cyproterone acetate on pituitary and testicular hormones in normal men". J. Clin. Endocrinol. Metab. 59 (5): 963–9. doi:10.1210/jcem-59-5-963. PMID 6237116.
- Jacobi GH, Altwein JE, Kurth KH, Basting R, Hohenfellner R (1980). "Treatment of advanced prostatic cancer with parenteral cyproterone acetate: a phase III randomised trial". Br J Urol. 52 (3): 208–15. doi:10.1111/j.1464-410x.1980.tb02961.x. PMID 7000222.
- Sander S, Nissen-Meyer R, Aakvaag A (1978). "On gestagen treatment of advanced prostatic carcinoma". Scand. J. Urol. Nephrol. 12 (2): 119–21. doi:10.3109/00365597809179977. PMID 694436.
- Kjeld JM, Puah CM, Kaufman B, Loizou S, Vlotides J, Gwee HM, Kahn F, Sood R, Joplin GF (1979). "Effects of norgestrel and ethinyloestradiol ingestion on serum levels of sex hormones and gonadotrophins in men". Clin. Endocrinol. (Oxf). 11 (5): 497–504. doi:10.1111/j.1365-2265.1979.tb03102.x. PMID 519881.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 390–. ISBN 978-1-4757-2085-3.
- Bińkowska M, Woroń J (2015). "Progestogens in menopausal hormone therapy". PRZ Menopauzalny. 14 (2): 134–43. doi:10.5114/pm.2015.52154. PMC 4498031. PMID 26327902.
- Mary C. Brucker; Tekoa L. King (8 September 2015). Pharmacology for Women's Health. Jones & Bartlett Publishers. pp. 368–. ISBN 978-1-284-05748-5.
- Donna Shoupe; Daniel R. Mishell, Jr. (28 September 2015). The Handbook of Contraception: A Guide for Practical Management. Humana Press. pp. 63–. ISBN 978-3-319-20185-6.
- Singh SM, Gauthier S, Labrie F (2000). "Androgen receptor antagonists (antiandrogens): structure-activity relationships". Curr. Med. Chem. 7 (2): 211–47. doi:10.2174/0929867003375371. PMID 10637363.
- Bergink EW, Loonen PB, Kloosterboer HJ (1985). "Receptor binding of allylestrenol, a progestagen of the 19-nortestosterone series without androgenic properties". J. Steroid Biochem. 23 (2): 165–8. doi:10.1016/0022-4731(85)90232-8. PMID 3928974.
- Ojasoo T, Delettré J, Mornon JP, Turpin-VanDycke C, Raynaud JP (1987). "Towards the mapping of the progesterone and androgen receptors". J. Steroid Biochem. 27 (1–3): 255–69. doi:10.1016/0022-4731(87)90317-7. PMID 3695484.
- Schubert K, Schumann G, Kaufmann G (1983). "Influence of a 9-double bond on stereospecific microbial 4,5-reductions". J. Steroid Biochem. 18 (1): 75–80. doi:10.1016/0022-4731(83)90333-3. PMID 6683343.
- Hobe G, Schön R, Hajek M, Undisz K, Härtl A (1998). "Studies on the hydrogenation of the progestagen dienogest in vivo and in vitro in the female rabbit". Steroids. 63 (7–8): 393–400. doi:10.1016/s0039-128x(98)00014-2. PMID 9654645.
- Oettel M, Kurischko A (1980). "STS 557, a new orally active progestin with antiprogestational and contragestational properties in rabbits". Contraception. 21 (1): 61–9. doi:10.1016/0010-7824(80)90140-7. PMID 7357870.
- http://www.micromedexsolutions.com/micromedex2/%5B%5D
- Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2094. ISBN 978-0-85369-840-1.
- Manetti GJ, Honig SC (2010). "Update on male hormonal contraception: is the vasectomy in jeopardy?". Int. J. Impot. Res. 22 (3): 159–70. doi:10.1038/ijir.2010.2. PMID 20336073.
- Misro MM, Chaki SP, Kaushik MC, Nandan D (June 2009). "Trials for development of once-a-month injectable, hormonal male contraceptive using dienogest plus testosterone undecanoate: dose standardization, efficacy and reversibility studies in rats". Contraception. 79 (6): 488–97. doi:10.1016/j.contraception.2009.01.003. PMID 19442786.
- "Estradiol valerate/dienogest - AdisInsight".
- Paslakis G, Maas S, Gebhardt B, Mayr A, Rauh M, Erim Y (April 2018). "Prospective, randomized, double-blind, placebo-controlled phase IIa clinical trial on the effects of an estrogen-progestin combination as add-on to inpatient psychotherapy in adult female patients suffering from anorexia nervosa". BMC Psychiatry. 18 (1): 93. doi:10.1186/s12888-018-1683-1. PMC 5891970. PMID 29631553.
Further reading
- Foster RH, Wilde MI (November 1998). "Dienogest". Drugs. 56 (5): 825–33, discussion 834–5. doi:10.2165/00003495-199856050-00007. PMID 9829156.
- Wellington K, Perry CM (2002). "Estradiol valerate/dienogest". Drugs. 62 (3): 491–504, discussion 505–6. doi:10.2165/00003495-200262030-00006. PMID 11827562.
- Teichmann A (August 2003). "Pharmacology of estradiol valerate/dienogest". Climacteric. 6 Suppl 2: 17–23. doi:10.0000/proquest/79ede894b4fb04720f1ebf0cfba74f80 (inactive 2019-12-14). PMID 14669840.
- von Schoultz B (August 2003). "Clinical efficacy and safety of combined estradiol valerate and dienogest: a new no-bleed treatment". Climacteric. 6 Suppl 2: 24–32. doi:10.0000/proquest/539296173d468a53486a72fb1229024b (inactive 2019-12-14). PMID 14669841.
- Sasagawa S, Shimizu Y, Imada K, Mizuguchi K (January 2009). "[Pharmacological and clinical profile of dienogest (DINAGEST Tab. 1 mg)]". Nippon Yakurigaku Zasshi (in Japanese). 133 (1): 32–40. doi:10.1254/fpj.133.32. PMID 19145049.
- Hoy SM, Scott LJ (August 2009). "Estradiol valerate/dienogest: in oral contraception". Drugs. 69 (12): 1635–46. doi:10.2165/11202820-000000000-00000. PMID 19678714.
- Harada T, Taniguchi F (January 2010). "Dienogest: a new therapeutic agent for the treatment of endometriosis". Womens Health (Lond). 6 (1): 27–35. doi:10.2217/whe.09.72. PMID 20001868.
- Jensen JT (May 2010). "Evaluation of a new estradiol oral contraceptive: estradiol valerate and dienogest". Expert Opin Pharmacother. 11 (7): 1147–57. doi:10.1517/14656561003724713. PMID 20367275.
- Pérez-Campos EF (April 2010). "Ethinylestradiol/dienogest in oral contraception". Drugs. 70 (6): 681–9. doi:10.2165/11536320-000000000-00000. PMID 20394455.
- "Estradiol + dienogest. Oral contraception: estradiol does not provide a therapeutic advantage". Prescrire Int. 19 (106): 65–7. April 2010. PMID 20568487.
- McCormack PL (November 2010). "Dienogest: a review of its use in the treatment of endometriosis". Drugs. 70 (16): 2073–88. doi:10.2165/11206320-000000000-00000. PMID 20964453.
- Keder LM (January 2011). "A new estradiol-dienogest oral contraceptive marks "The Pill's" 50th anniversary". Am J Ther. 18 (1): 38–44. doi:10.1097/MJT.0b013e3182068cc6. PMID 21192241.
- Whalen KL, Rose R (October 2011). "Estradiol valerate/dienogest: a novel oral contraceptive". Ann Pharmacother. 45 (10): 1256–61. doi:10.1345/aph.1Q216. PMID 21917554.
- Borgelt LM, Martell CW (January 2012). "Estradiol valerate/dienogest: a novel combined oral contraceptive". Clin Ther. 34 (1): 37–55. doi:10.1016/j.clinthera.2011.11.006. PMID 22169052.
- Ruan X, Seeger H, Mueck AO (April 2012). "The pharmacology of dienogest". Maturitas. 71 (4): 337–44. doi:10.1016/j.maturitas.2012.01.018. PMID 22364708.
- Fruzzetti F, Trémollieres F, Bitzer J (May 2012). "An overview of the development of combined oral contraceptives containing estradiol: focus on estradiol valerate/dienogest". Gynecol. Endocrinol. 28 (5): 400–8. doi:10.3109/09513590.2012.662547. PMC 3399636. PMID 22468839.
- Micks EA, Jensen JT (January 2013). "Treatment of heavy menstrual bleeding with the estradiol valerate and dienogest oral contraceptive pill". Adv Ther. 30 (1): 1–13. doi:10.1007/s12325-012-0071-3. PMID 23239397.
- Bizzarri N, Remorgida V, Leone Roberti Maggiore U, Scala C, Tafi E, Ghirardi V, Salvatore S, Candiani M, Venturini PL, Ferrero S (September 2014). "Dienogest in the treatment of endometriosis". Expert Opin Pharmacother. 15 (13): 1889–902. doi:10.1517/14656566.2014.943734. PMID 25069386.
- Nappi RE, Serrani M, Jensen JT (2014). "Noncontraceptive benefits of the estradiol valerate/dienogest combined oral contraceptive: a review of the literature". Int J Women's Health. 6: 711–8. doi:10.2147/IJWH.S65481. PMC 4128844. PMID 25120376.
- Graziottin A (October 2014). "Contraception containing estradiol valerate and dienogest--advantages, adherence and user satisfaction". Minerva Ginecol. 66 (5): 479–95. PMID 25245997.
- Agarwal S, Fraser MA, Chen I, Singh SS (February 2015). "Dienogest for the treatment of deep endometriosis: case report and literature review". J. Obstet. Gynaecol. Res. 41 (2): 309–13. doi:10.1111/jog.12527. PMID 25303112.
- Andres Mde P, Lopes LA, Baracat EC, Podgaec S (September 2015). "Dienogest in the treatment of endometriosis: systematic review". Arch. Gynecol. Obstet. 292 (3): 523–9. doi:10.1007/s00404-015-3681-6. PMID 25749349.
- Ferrero S, Remorgida V, Venturini PL, Bizzarri N (June 2015). "Endometriosis: the effects of dienogest". BMJ Clin Evid. 2015. PMC 4461025. PMID 26057101.
- "Dienogest". Aust Prescr. 38 (4): 138–9. August 2015. doi:10.18773/austprescr.2015.050. PMC 4653971. PMID 26648643.
- Grandi G, Mueller M, Bersinger NA, Cagnacci A, Volpe A, McKinnon B (March 2016). "Does dienogest influence the inflammatory response of endometriotic cells? A systematic review". Inflamm. Res. 65 (3): 183–92. doi:10.1007/s00011-015-0909-7. PMID 26650031.
- Laganà AS, Vitale SG, Muscia V, Rossetti P, Buscema M, Triolo O, Rapisarda AM, Giunta L, Palmara V, Granese R, Frangež HB, Romano A (March 2017). "Endometrial preparation with Dienogest before hysteroscopic surgery: a systematic review". Arch. Gynecol. Obstet. 295 (3): 661–667. doi:10.1007/s00404-016-4244-1. PMID 27904953.
- Bedaiwy MA, Allaire C, Alfaraj S (March 2017). "Long-term medical management of endometriosis with dienogest and with a gonadotropin-releasing hormone agonist and add-back hormone therapy". Fertil. Steril. 107 (3): 537–548. doi:10.1016/j.fertnstert.2016.12.024. PMID 28139239.
- García Uranga-Romano J, Hernández-Valencia M, Zárate A, Basavilvazo-Rodríguez MA (2017). "[Dienogest usefulness in pelvic pain due to endometriosis. A meta-analysis of its effectiveness]". Rev Med Inst Mex Seguro Soc (in Spanish). 55 (4): 452–455. PMID 28591499.
- Maiorana A, Incandela D, Parazzini F, Alio W, Mercurio A, Giambanco L, Alio L (September 2017). "Efficacy of dienogest in improving pain in women with endometriosis: a 12-month single-center experience". Arch. Gynecol. Obstet. 296 (3): 429–433. doi:10.1007/s00404-017-4442-5. PMID 28664483.
- Regidor PA, Schindler AE (October 2017). "Antiandrogenic and antimineralocorticoid health benefits of COC containing newer progestogens: dienogest and drospirenone". Oncotarget. 8 (47): 83334–83342. doi:10.18632/oncotarget.19833. PMC 5669973. PMID 29137347.