Apararenone
Apararenone (INN) (developmental code name MT-3995) is a nonsteroidal antimineralocorticoid which is under development by Mitsubishi Tanabe Pharma for the treatment of diabetic nephropathies and non-alcoholic steatohepatitis.[1][2][3] It was also previously being developed for the treatment of hypertension, but development was discontinued for this indication.[1] Apararenone acts as a highly selective antagonist of the mineralocorticoid receptor (Ki < 50 nM), the receptor for aldosterone.[1][2][3] As of 2017, it is in phase II clinical trials.[1]
 | |
| Clinical data | |
|---|---|
| Other names | MT-3995 |
| Routes of administration | Oral |
| Drug class | Antimineralocorticoid |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
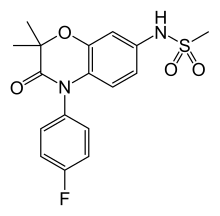
| Formula | C17H17FN2O4S |
| Molar mass | 364.391 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- http://adisinsight.springer.com/drugs/800032686
- Yang J, Young MJ (2016). "Mineralocorticoid receptor antagonists-pharmacodynamics and pharmacokinetic differences". Curr Opin Pharmacol. 27: 78–85. doi:10.1016/j.coph.2016.02.005. PMID 26939027.
- Kolkhof P, Nowack C, Eitner F (2015). "Nonsteroidal antagonists of the mineralocorticoid receptor". Curr. Opin. Nephrol. Hypertens. 24 (5): 417–24. doi:10.1097/MNH.0000000000000147. PMID 26083526.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.