JNJ-39393406
JNJ-39393406 is an experimental medication which is under development by Janssen Pharmaceutica, a division of Johnson & Johnson, for the treatment of depressive disorders and smoking withdrawal.[1] It acts as a selective positive allosteric modulator of the α7 nicotinic acetylcholine receptor (nAChR).[1] It does not act on the α4β2 or α3β4 nAChRs or the serotonin 5-HT3 receptor, and does not interact with a panel of 62 other receptors and enzymes.[2] The drug has been found to lower the agonist and nicotine threshold for activation of the α7 nAChR by 10- to 20-fold and to increase the maximum agonist response of the α7 nAChR by 17- to 20-fold.[2] As of February 2018, JNJ-39393406 is in phase II clinical trials for both depressive disorders and smoking withdrawal.[1] It was also under investigation for the treatment of schizophrenia and Alzheimer's disease, but development for these indications was discontinued.[1]
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth[1] |
| Drug class | Antinicotinic |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
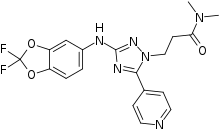
| Formula | C19H18F2N6O3 |
| Molar mass | 416.389 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- "JNJ-39393406". Adis Insight.
- Winterer G, Gallinat J, Brinkmeyer J, Musso F, Kornhuber J, Thuerauf N, Rujescu D, Favis R, Sun Y, Franc MA, Ouwerkerk-Mahadevan S, Janssens L, Timmers M, Streffer JR (2013). "Allosteric alpha-7 nicotinic receptor modulation and P50 sensory gating in schizophrenia: a proof-of-mechanism study". Neuropharmacology. 64: 197–204. doi:10.1016/j.neuropharm.2012.06.040. PMID 22766391.