Gestronol
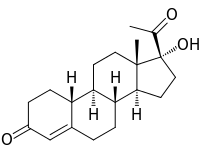
Gestronol (BAN), also known as gestonorone, as well as 17α-hydroxy-19-norprogesterone or 17α-hydroxy-19-norpregn-4-ene-3,20-dione, is a progestin of the 19-norprogesterone and 17α-hydroxyprogesterone groups which was never marketed.[1][2][3] The C17α caproate ester of gestronol, gestonorone caproate (gestronol hexanoate), in contrast, has been marketed.[1][2][3]
 | |
| Clinical data | |
|---|---|
| Other names | Gestonorone; 17α-Hydroxy-19-norprogesterone; 17α-Hydroxy-19-norpregn-4-ene-3,20-dione |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.016.708 |
| Chemical and physical data | |
| Formula | C20H28O3 |
| Molar mass | 316.441 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Gestronol shows relatively low affinity for the progesterone receptor, only about 12.5% of that of progesterone and about 2.5% of that of 19-norprogesterone in one assay.[4] On the other hand, gestronol had far higher affinity than 17α-hydroxyprogesterone, which showed less than 0.1% of the affinity of progesterone for the progesterone receptor.[4]
See also
References
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 595–. ISBN 978-1-4757-2085-3.
- I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 132–. ISBN 978-94-011-4439-1.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 1357–. ISBN 978-3-88763-075-1.
- Botella J, Duc I, Delansorne R, Paris J, Lahlou B (December 1990). "Structure-activity and structure-affinity relationships of 19-nor-progesterone derivatives in rat uterus". J. Endocrinol. Invest. 13 (11): 905–10. doi:10.1007/BF03349652. PMID 2090671.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.