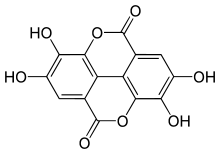
Ellagic acid
Ellagic acid is a natural phenol antioxidant found in numerous fruits and vegetables. The antiproliferative and antioxidant properties of ellagic acid have prompted research into its potential health benefits. Ellagic acid is the dilactone of hexahydroxydiphenic acid.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2,3,7,8-Tetrahydroxy-chromeno[5,4,3-cde]chromene-5,10-dione | |
| Other names
4,4′,5,5′,6,6′-Hexahydroxydiphenic acid 2,6,2′,6′-dilactone | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.006.827 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C14H6O8 |
| Molar mass | 302.197 g/mol |
| Density | 1.67 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Name
The name comes from the French term acide ellagique, from the word galle spelled backwards[1] because it can be obtained from noix de galle (galls), and to distinguish it from acide gallique (gallic acid).
Metabolism
Biosynthesis
Plants produce ellagic acid from hydrolysis of tannins such as ellagitannin and geraniin.[2]
Biodegradation
Urolithins are microflora human metabolites of dietary ellagic acid derivatives[3]
History
Ellagic acid was first discovered by chemist Henri Braconnot in 1831.[4] Maximilian Nierenstein prepared this substance from algarobilla, dividivi, oak bark, pomegranate, myrabolams, and valonea in 1905.[4] He also suggested its formation from galloyl-glycine by Penicillium in 1915.[5] Julius Löwe was the first person to synthesize ellagic acid by heating gallic acid with arsenic acid or silver oxide.[4][6]
Natural occurrences
Ellagic acid is found in oak species such as the North American white oak (Quercus alba) and European red oak (Quercus robur).[7]
The macrophyte Myriophyllum spicatum produces ellagic acid.[8]
Ellagic acid can be found in the medicinal mushroom Phellinus linteus.[9]
Medicinal claims and research
Ellagic acid has been marketed as a dietary supplement with a range of claimed benefits against cancer, heart disease, and other medical problems. Ellagic acid has been identified by the U.S. Food and Drug Administration as a "fake cancer 'cure'".[13] A number of U.S.-based sellers of dietary supplements have received Warning Letters from the Food and Drug Administration for promoting ellagic acid with claims that violate the Federal Food, Drug, and Cosmetic Act.[14][15]
Urolithins, such as urolithin A, are microflora human metabolites of dietary ellagic acid derivatives that are under study as anti-cancer agents.[16] Claims that ellagic acid can treat or prevent cancer in humans have not been proven.[17]
References
- Littré, Émile. "ellagique". Dictionnaire de la langue française.
- Seigler, David S. (31 December 1998). Plant Secondary Metabolism. Springer Science & Business Media. p. 208. ISBN 978-0-412-01981-4.
- Larrosa, M.; González Sarrías, A.; García Conesa, M. T.; Tomás Barberán, F. A.; Espín, J. C. (2006). "Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities". Journal of Agricultural and Food Chemistry. 54 (5): 1611–1620. doi:10.1021/jf0527403. PMID 16506809.
- Grasser, Georg; Enna, F. G. A. (1922). Synthetic Tannins. p. 20. ISBN 9781406773019.
- Nierenstein, M. (1915). "The Formation of Ellagic Acid from Galloyl-Glycine by Penicillium". The Biochemical Journal. 9 (2): 240–244. doi:10.1042/bj0090240. PMC 1258574. PMID 16742368.
- Löwe, Julius (1868). "Über die Bildung von Ellagsäure aus Gallussäure" [On the synthesis of ellagic acid from gallic acid]. Zeitschrift für Chemie. 4: 603.
- Mämmelä, P.; Savolainen, H.; Lindroos, L.; Kangas, J.; Vartiainen, T. (2000). "Analysis of oak tannins by liquid chromatography-electrospray ionisation mass spectrometry". Journal of Chromatography A. 891 (1): 75–83. doi:10.1016/S0021-9673(00)00624-5. PMID 10999626.
- Nakai, S. (2000). "Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa". Water Research. 34 (11): 3026–3032. doi:10.1016/S0043-1354(00)00039-7.
- Lee, Y.-S.; Kang, Y.-H.; Jung, J.-Y.; Lee, S.; Ohuchi, K.; Shin, K.-H.; Kang, I.-J.; Park, J.-H.; Shin, H.-K.; Lim, S.-S. (2008). "Protein glycation inhibitors from the fruiting body of Phellinus linteus". Biological and Pharmaceutical Bulletin. 31 (10): 1968–1972. doi:10.1248/bpb.31.1968. PMID 18827365.
- Vattem, D. A.; Shetty, K. (2005). "Biological Function of Ellagic Acid: A Review". Journal of Food Biochemistry. 29 (3): 234–266. doi:10.1111/j.1745-4514.2005.00031.x.
- Infante, R.; Contador, L.; Rubio, P.; Aros, D.; Peña Neira, Á. (2011). "Postharvest sensory and phenolic characterization of 'Elegant Lady' and 'Carson' peaches" (PDF). Chilean Journal of Agricultural Research. 71 (3): 445–451. doi:10.4067/S0718-58392011000300016.
- Usta, C.; Özdemir, S.; Schiariti, M.; Puddu, P. E. (November 2013). "The pharmacological use of ellagic acid-rich pomegranate fruit". International Journal of Food Sciences and Nutrition. 64 (7): 907–913. doi:10.3109/09637486.2013.798268. PMID 23700985.
- "187 Fake Cancer 'Cures' Consumers Should Avoid". U.S. Food and Drug Administration. Archived from the original on May 2, 2017. Retrieved June 17, 2008.
- "Warning Letter sent to Millennium Health". Food and Drug Administration. May 21, 2008.
- "Warning Letter sent to Kenton Campbell at Prime Health Direct, Ltd" (PDF). Food and Drug Administration. July 2, 2007.
- Davis, C. D.; Milner, J. A. (2009). "Gastrointestinal microflora, food components and colon cancer prevention". Journal of Nutritional Biochemistry. 20 (10): 743–52. doi:10.1016/j.jnutbio.2009.06.001. PMC 2743755. PMID 19716282.
- "Ellagic acid". American Cancer Society. November 2008. Retrieved August 1, 2014.