Estrogen patch
The estrogen patch (oestrogen patch) is a delivery system for estradiol, which is used as hormone replacement therapy to treat the problems of menopause, such as hot flashes and vaginal dryness, or to prevent osteoporosis, as well as in hormone replacement therapy for transgender women. The estrogen is given transdermally rather than via oral tablets, meaning that the estrogen patch carries similar risks and benefits that conventional forms of estrogen-only hormone replacement therapy have, but there are also important differences.

The route of administration of estrogens may have implications for adverse effects. For example, transdermal estrogen bypasses the liver so avoids the liver effects that occur with use of oral medications, and has slightly different effects on triglycerides and cholesterol than oral estrogens. Also, the specific type of estrogen is of importance, with transdermal 17-beta estradiol not having the increased risk of venous thromboembolism seen with ethinyl estradiol.
Administration
Applied twice weekly or weekly, depending on the brand, to fatty areas of the skin which crease less, preferably the lower abdomen or buttocks. Never to the breasts.
Formulations
| Brand name |
Forms (µg/day) | Duration | Type | Size (cm2)a | Estradiol (mg) | Levels (pg/mL) |
Launch (year) |
Hits | |
|---|---|---|---|---|---|---|---|---|---|
| Alora | 25, 50, 75, 100 | 3–4 days | Matrix | 9, 18, 27, 36 | 0.77, 1.5, 2.3, 3.1 | 43–144 | 1996 | 42,300 | |
| Climara | 25, 37.5, 50, 60, 75, 100 | 7 days | Matrix | 6.5, 9.375, 12.5, 15, 18.75, 25 | 2, 2.85, 3.8, 4.55, 5.7, 7.6 | 17–174 | 1994 | 110,000 | |
| Climara Prob | E2 (45) + LNG (15) | 7 days | Matrix | 22 | 4.4 | 27–54 | 2003 | 23,400 | |
| CombiPatchb | E2 (50) + NETA (14, 25) | 3–4 days | Matrix | 9, 16 | 0.62, 0.51 | 27–71 | 1998 | 33,500 | |
| Estradiolc | 25, 37.5, 50, 75, 100 | 3–4 days | Matrix | 2.5, 3.75, 5, 7.5, 10 | 0.41, 0.62, 0.82, 1.23, 1.64 | 30–145 | 1996 | – | |
| Estradiolc | 25, 37.5, 50, 75, 100 | 7 days | Matrix | 7.75, 11.625, 15.5, 18.6, 23.25, 31 | 0.97, 1.46, 1.94, 2.33, 2.91, 3.88 | 17–174 | 2000 | – | |
| Menostar | 14 | 7 days | Matrix | 3.25 | 1 | 13–21 | 2004 | 21,300 | |
| Minivelle | 25, 37.5, 50, 75, 100 | 3–4 days | Matrix | 1.65, 2.48, 3.3, 4.95, 6.6 | 0.41, 0.62, 0.83, 1.24, 1.65 | 30–117 | 2012 | 15,100 | |
| Vivelle | 3–4 days | Matrix | 30–145 | 2000 | 91,900 | ||||
| Vivelle-Dot | 25, 37.5, 50, 75, 100 | 3–4 days | Matrix | 2.5, 3.75, 5, 7.5, 10 | 0.39, 0.585, 0.78, 1.17, 1.56 | 30–145 | 1996 | 68,900 | |
| Abbreviations: E2 = Estradiol. LNG = Levonorgestrel. NETA = Norethisterone acetate. EtOH = Ethanol. Notes: | |||||||||
Estrogen levels
_in_postmenopausal_women.png)
_in_postmenopausal_women.png)
_in_women.png) Levels of estradiol over a period of 8 days after a single application of a 50 or 100 μg/day Climara-type (Climara, Menostar, Mylan generic) once-weekly transdermal estradiol matrix patch to the abdomen and removed on day 7 in postmenopausal women.[7]
Levels of estradiol over a period of 8 days after a single application of a 50 or 100 μg/day Climara-type (Climara, Menostar, Mylan generic) once-weekly transdermal estradiol matrix patch to the abdomen and removed on day 7 in postmenopausal women.[7]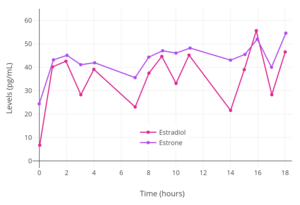 Levels of estradiol and estrone with application of a single 50 µg/day estradiol transdermal reservoir patch (Estraderm) in postmenopausal women.[8]
Levels of estradiol and estrone with application of a single 50 µg/day estradiol transdermal reservoir patch (Estraderm) in postmenopausal women.[8]_with_and_without_an_ethanol_injection_in_postmenopausal_women.png) Levels of estradiol over the course of 15 days with a single application of a 100 µg/day estradiol transdermal reservoir patch (Estraderm) in postmenopausal women.[9] This patch has a 3- to 4-day duration and is designed for twice-weekly use. Patch was applied on day 1 and removed on day 7.[9] In one group, ethanol was injected into the area between the patch and the skin on day 3 to restore the solvent, and resulted in increased bioavailability and a prolonged duration of the patch.[9]
Levels of estradiol over the course of 15 days with a single application of a 100 µg/day estradiol transdermal reservoir patch (Estraderm) in postmenopausal women.[9] This patch has a 3- to 4-day duration and is designed for twice-weekly use. Patch was applied on day 1 and removed on day 7.[9] In one group, ethanol was injected into the area between the patch and the skin on day 3 to restore the solvent, and resulted in increased bioavailability and a prolonged duration of the patch.[9]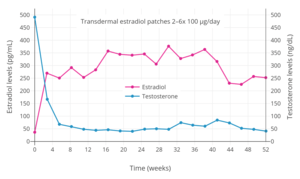
Brand names
The following are brand names of estradiol patches in the United States and United Kingdom:[13][14]
- United States
- Alora
- Climara
- Climara Pro (with levonorgestrel)
- CombiPatch (with norethisterone acetate)
- Elleste Solo
- Esclim (discontinued)
- Estraderm (discontinued)
- FemPatch
- Menostar
- Minivelle
- Vivelle
- Vivelle-Dot
- United Kingdom
- Dermestril
- Elleste Solo MX
- Estracombi (with norethisterone acetate)
- Estraderm MX
- Estraderm TTS
- Estradot
- Estrapak (pack with oral norethisterone acetate tablets)
- Evorel
- Evorel Conti (with norethisterone acetate)
- Evorel-Pak (pack with oral norethisterone acetate tablets)
- Femapak (pack with oral dydrogesterone tablets)
- Fematrix
- FemSeven
- Nuvelle TS (with cyclical levonorgestrel)
- Progynova TS
Patches with progestogens
Some patch systems for hormone replacement therapy consist of a continuous estrogen patch in addition to an intermittent patch containing a progestogen (progestin), in order to decrease disturbances in the endometrium of the uterus. The combination of estrogen and progestogen make them somewhat similar to the contraceptive patch. For example, Sequidot consists of a continuous estradiol patch in addition to a norethisterone patch that is worn 14 days in each 28-day cycle.[15]
References
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20323S23lbl.pdf
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020538s035lbl.pdf
- https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d28bec8f-762e-4f05-a20d-96a42970d6a7
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020375s034lbl.pdf
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020375s035lbl.pdf
- https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e7e6da3b-8485-1382-61c9-e9b369018b98
- "Climara Forte" (HTML). HPRA. Retrieved 22 December 2018.
- Vinod P. Shah; Howard I. Maibach (29 June 2013). Topical Drug Bioavailability, Bioequivalence, and Penetration. Springer Science & Business Media. pp. 47–50. ISBN 978-1-4899-1262-6.
- C. Christian; B. von Schoultz (15 March 1994). Hormone Replacement Therapy: Standardized or Individually Adapted Doses?. CRC Press. pp. 9–16, 60. ISBN 978-1-85070-545-1.
The mean integrated estradiol level during a full 28-day normal cycle is around 80 pg/ml.
- Ockrim JL, Lalani EN, Laniado ME, Carter SS, Abel PD (May 2003). "Transdermal estradiol therapy for advanced prostate cancer--forward to the past?". J. Urol. 169 (5): 1735–7. doi:10.1097/01.ju.0000061024.75334.40. PMID 12686820.
- Langley RE, Godsland IF, Kynaston H, Clarke NW, Rosen SD, Morgan RC, Pollock P, Kockelbergh R, Lalani EN, Dearnaley D, Parmar M, Abel PD (August 2008). "Early hormonal data from a multicentre phase II trial using transdermal oestrogen patches as first-line hormonal therapy in patients with locally advanced or metastatic prostate cancer". BJU Int. 102 (4): 442–5. doi:10.1111/j.1464-410X.2008.07583.x. PMC 2564109. PMID 18422771.
- Ockrim J, Lalani EN, Abel P (October 2006). "Therapy Insight: parenteral estrogen treatment for prostate cancer--a new dawn for an old therapy". Nat Clin Pract Oncol. 3 (10): 552–63. doi:10.1038/ncponc0602. PMID 17019433.
- "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 5 May 2019.
- https://www.earlymenopause.com/information/uk-hrt/
- Drugs.com > Sequidot. Retrieved March 2014
External links
- Vivelle Dot official website at the Library of Congress Web Archives (archived 2011-03-02)
-solution.jpg)

