5-Methyl-MDA
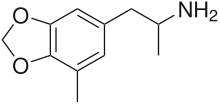
5-Methyl-3,4-methylenedioxyamphetamine (5-Methyl-MDA) is an entactogen and psychedelic designer drug of the amphetamine class. It is a ring-methylated homologue of MDA and a structural isomer of MDMA.[1]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| ChEMBL | |
| Chemical and physical data | |
| Formula | C11H15NO2 |
| Molar mass | 193.242 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Effects and research
Drug discrimination studies showed that 5-methyl-MDA substitutes for MDA, MMAI, and LSD, but not amphetamine, suggesting that it produces a mix of entactogen and hallucinogenic effects without any stimulant effects.
5-Methyl-MDA acts as a selective serotonin releasing agent (SSRA) with IC50 values of 107nM, 11,600nM, and 1,494nM for serotonin, dopamine, and norepinephrine efflux.[1] It is over 5 times more potent than MDA in vitro assays, with a suitable active dose possibly in vivo being around 15–25 mg.[1] Subsequent testing in vivo, however, has found that it is not as potent as once thought and is active at at least 100 mg. 2-Methyl-MDA is also much more potent than MDA, but is not quite as potent as 5-methyl-MDA.[1] 6-methyl-MDMA (also known as Madam-6) is mostly inactive, likely due to steric hindrance.[1][2]
Recent research has used data on 2-methyl-MDA and 5-methyl-MDA to help guide computer modeling of the serotonin transporter complex.[3]
Legal status
5-Methyl-MDA is not scheduled by the United Nations' Convention on Psychotropic Substances.[4]
References
- Parker MA, Marona-Lewicka D, Kurrasch D, Shulgin AT, Nichols DE (March 1998). "Synthesis and pharmacological evaluation of ring-methylated derivatives of 3,4-(methylenedioxy)amphetamine (MDA)". Journal of Medicinal Chemistry. 41 (6): 1001–5. doi:10.1021/jm9705925. PMID 9526575.
- PIHKAL #98
- Walline CC, Nichols DE, Carroll FI, Barker EL (June 2008). "Comparative molecular field analysis using selectivity fields reveals residues in the third transmembrane helix of the serotonin transporter associated with substrate and antagonist recognition". The Journal of Pharmacology and Experimental Therapeutics. 325 (3): 791–800. doi:10.1124/jpet.108.136200. PMC 2637348. PMID 18354055.
- Convention on Psychotropic Substances, 1971
- §1308.11 Schedule I.
- Erowid Analog Law Vault : Federal Controlled Substance Analogue Act Summary
Empathogens/entactogens | |
|---|---|
| Phenylalkyl- amines (other than cathinones) |
|
| Cyclized phenyl- alkylamines | |
| Cathinones |
|
| Tryptamines | |
| Chemical classes | |
| DRAs |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAs |
| ||||||||||||||
| SRAs |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine reuptake inhibitors • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|