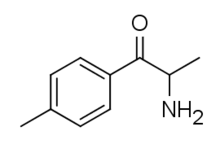
4-Methylcathinone
4-Methylcathinone (also known as Nor-Mephedrone, 4-MC and NSC-60487), is a stimulant drug of the cathinone chemical class that has been sold online as a designer drug.[1] It is a metabolite of the better known drug mephedrone (4-methylmethcathinone).[2][3][4]
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C10H13NO |
| Molar mass | 163.219 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
4-Methylcathinone displays a 2.4-fold selectivity to promote monoamine release via DAT over SERT as opposed to 309-fold selectivity for cathinone.[5]
See also
References
- "nor-Mephedrone". Cayman Chemical. Retrieved 27 June 2015.
- Markus R. Meyer; Jens Wilhelm; Frank T. Peters; Hans H. Maurer (June 2010). "Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography–mass spectrometry". Analytical and Bioanalytical Chemistry. 397 (3): 1225–1233. doi:10.1007/s00216-010-3636-5. PMID 20333362.
- Anders Just Pedersen; Lotte Ask Reitzel; Sys Stybe Johansen; Kristian Linnet (June 2013). "In vitro metabolism studies on mephedrone and analysis of forensic cases". Drug Testing and Analysis. 5 (6): 430–438. doi:10.1002/dta.1369. PMID 22573603.
- Paul I. Dargan; Roumen Sedefov; Ana Gallegos; Dr David M. Wood (July–August 2011). "The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone)". Drug Testing and Analysis. 3 (7–8): 454–463. doi:10.1002/dta.312. PMID 21755604.
- Blake A. Hutsell; Michael H. Baumann; John S. Partilla; Matthew L. Banks; Rakesh Vekariya; Richard A. Glennon; S. Stevens Negus (February 2016). "Abuse-related neurochemical and behavioral effects of cathinone and 4-methylcathinone stereoisomers in rats". European Neuropsychopharmacology. 26 (2): 288–297. doi:10.1016/j.euroneuro.2015.12.010. PMC 5331761. PMID 26738428.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.