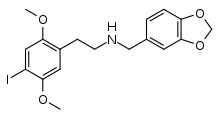
25I-NB34MD
25I-NB34MD (NB34MD-2C-I) is a derivative of the phenethylamine hallucinogen 2C-I, which acts as a potent partial agonist for the human 5-HT2A receptor, and presumably has similar properties to 2C-I.[1] It has a binding affinity of 0.67nM at the human 5-HT2A receptor, making it several times weaker than its positional isomer 25I-NBMD and a similar potency to 25I-NBF.[2][3]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| ChemSpider |
|
| Chemical and physical data | |
| Formula | C18H20INO4 |
| Molar mass | 441.259 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Legality
Hungary
Illegal.[4]
Japan
Illegal.[5]
Sweden
The Riksdag added 25I-NB34MD to Narcotic Drugs Punishments Act under swedish schedule I ("substances, plant materials and fungi which normally do not have medical use") as of June 9, 2015, published by Medical Products Agency (MPA) in regulation LVFS 2015:4 listed as 25I-NB34MD, and 2-(4-jodo-2,5-dimetoxifenyl)-N-[(3,4-metylendioxifenyl)metyl]etanamin.[6]
United Kingdom
This substance is a Class A drug in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause in the Misuse of Drugs Act 1971.[7]
Analogues and derivatives
Analogues and derivatives of 2C-I:
25I-NB*:
- 25I-NBF
- 25I-NBMD
- 25I-NB34MD
- 25I-NBOH
- 25I-NBOMe (NBOMe-2CI)
- 25I-NB3OMe
- 25I-NB4OMe
References
- Nahoko Uchiyama; Ruri Kikura-Hanajiri; Takashi Hakamatsuka (January 2016). "A phenethylamine derivative 2-(4-iodo-2,5-dimethoxyphenyl)-N-[(3,4-methylenedioxyphenyl)methyl]ethanamine (25I-NB34MD) and a piperazine derivative 1-(3,4-difluoromethylenedioxybenzyl)piperazine (DF-MDBP), newly detected in illicit products". Forensic Toxicology. 34 (1): 166–173. doi:10.1007/s11419-015-0304-7.
- Braden, MR; Parrish, JC; Naylor, JC; Nichols, DE (December 2006). "Molecular Interaction of Serotonin 5-HT2A Receptor Residues Phe339(6.51) and Phe340(6.52) with Superpotent N-Benzyl Phenethylamine Agonists". Molecular Pharmacology. 70 (6): 1956–1964. doi:10.1124/mol.106.028720. PMID 17000863.
- Michael Robert Braden (2007). "Towards a biophysical understanding of hallucinogen action". Dissertation: 1–176.
- A Magyarországon megjelent, a Kábítószer és Kábítószer-függőség Európai Megfigyelő Központjának Korai Jelzőrendszerébe (EMCDDA EWS) 2005 óta bejelentett ellenőrzött anyagok büntetőjogi vonatkozású besorolása
- "指定薬物名称・構造式一覧(平成27年9月16日現在)" (PDF) (in Japanese). 厚生労働省. 16 September 2015. Retrieved 8 October 2015.
- https://lakemedelsverket.se/upload/lvfs/LVFS_2015_4.pdf
- "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". www.legislation.gov.uk.
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|