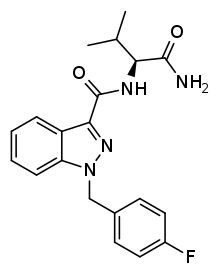
AB-FUBINACA
AB-FUBINACA is a drug that acts as a potent agonist for the cannabinoid receptors, with Ki values of 0.9 nM at CB1 and 23.2 nM at CB2 and EC50 values of 1.8 nM at CB1 and 3.2 nM at CB2.[1][2][3] It was originally developed by Pfizer in 2009 as an analgesic medication[4] but was never pursued for human use. In 2012, it was discovered as an ingredient in synthetic cannabinoid blends in Japan,[5] along with a related compound AB-PINACA, which had not previously been reported.
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H21FN4O2 |
| Molar mass | 368.412 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Its use has been linked to hospitalizations and deaths.[6][7]
Legality
It was designated as a Schedule I controlled substance in the United States in January 2014.[8]
It is an Anlage II controlled substance in Germany as of November 2014.[9]
It is a controlled substance in China as of October 2015.[10]
Mass overdoses
On August 15th, 2018, 70 people within the city of New Haven, Connecticut started overdosing near Yale University campus.[11] By the end of the week, the total number of overdosed had risen to over 100 people needing transport to local emergency rooms. Three men were arrested, charged as drug dealers selling synthetic cannabis which contained AB-FUBINACA.[12][13][14] Almost all of the overdoses occurred on the New Haven Green, a large downtown park that is heavily traveled and very popular with the homeless population. There have been no deaths associated with these overdoses; however, several victims are in critical or life-threatening condition.[15]
During a similar period, AB-FUBINACA was implicated in the hospitalization of hundreds of people in two cities in New Zealand; Napier and Christchurch.[16] There were over forty deaths in New Zealand as a result of synthetic cannabinoids in 2018, and dozens of people are still in intensive care. The New Zealand Drug Foundation has issued a national emergency as a result.[17]
See also
References
- Samuel D Banister; Michael Moir; Jordyn Stuart; Richard C Kevin; Katie E Wood; Mitchell Longworth; Shane M Wilkinson; Corinne Beinat; Alxendra S Buchanan; Michelle Glass; Mark Connor; Iain S McGregor; Michael Kassiou (July 2015). "The pharmacology of indole and indazole synthetic cannabinoid designer drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA and 5F-ADBICA". ACS Chemical Neuroscience. 6 (9): 1546–1559. doi:10.1021/acschemneuro.5b00112. PMID 26134475.
- Hsin-Hung Chen; Aybike Dip; Mostafa Ahmed; Michael L. Tan; Jeffrey P. Walterscheid; Hua Sun; Ba-Bie Teng; Ashraf Mozayani (April 2016). "Detection and Characterization of the Effect of AB-FUBINACA and its Metabolites in a Rat Model". Journal of Cellular Biochemistry. 117 (4): 1033–1043. doi:10.1002/jcb.25421. PMC 5063098. PMID 26517302.
- Svante Vikingsson; Henrik Gréen; Linda Brinkhagen; Shahzabe Mukhtar; Martin Josefsson (November 2015). "Identification of AB-FUBINACA metabolites in authentic urine samples suitable as urinary markers of drug intake using liquid chromatography quadrupole tandem time of flight mass spectrometry". Drug Testing and Analysis. 8 (9): 950–6. doi:10.1002/dta.1896. PMID 26560240.
- Buchler IP et al., INDAZOLE DERIVATIVES. WO 2009/106982
- Uchiyama, N.; Matsuda, S.; Wakana, D.; Kikura-Hanajiri, R.; Goda, Y. (January 2013). "New cannabimimetic indazole derivatives, N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide (AB-PINACA) and N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide (AB-FUBINACA) identified as designer drugs in illegal products". Forensic Toxicology. 31 (1): 93–100. doi:10.1007/s11419-012-0171-4.
- Jordan Trecki; Roy R. Gerona; Michael D. Schwartz (July 2015). "Synthetic Cannabinoid–Related Illnesses and Deaths". New England Journal of Medicine. 373 (2): 103–107. doi:10.1056/NEJMp1505328. PMID 26154784.
- Janez Klavž; Maksimiljan Gorenjak; Martin Marinšek (August 2016). "Suicide attempt with a mix of synthetic cannabinoids and synthetic cathinones: Case report of non-fatal intoxication with AB-CHMINACA, AB-FUBINACA, alpha-PHP, alpha-PVP and 4-CMC". Forensic Science International. 265: 121–124. doi:10.1016/j.forsciint.2016.01.018. PMID 26890319.
- "Schedules of Controlled Substances: Temporary Placement of Four Synthetic Cannabinoids Into Schedule I". Drug Enforcement Administration, Department of Justice. 10 January 2014. Retrieved 21 July 2015.
- "Gesetz über den Verkehr mit Betäubungsmitteln (Betäubungsmittelgesetz - BtMG) Anlage II (zu § 1 Abs. 1) (verkehrsfähige, aber nicht verschreibungsfähige Betäubungsmittel)" (in German). Retrieved 22 June 2015.
- "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015.
- Winston, Ali (2018-08-15). "New Haven Overdoses Tied to Laced K2". The New York Times.
- "Police Arrest Third Suspect In New Haven Synthetic Marijuana Overdose Case". NPR. 17 August 2018. Retrieved 20 August 2018.
- "Dozens Overdose In Connecticut Park On Tainted Synthetic Marijuana". NPR. 16 August 2018. Retrieved 20 August 2018.
- "New Haven police make arrest after more than 100 K2 overdose calls". CNN. 18 August 2018. Retrieved 18 August 2018.
- "Police Arrest Third Suspect In New Haven Synthetic Marijuana Overdose Case". NPR. 17 August 2018. Retrieved 20 August 2018.
- "Synthetic cannabis users gambling with their lives after a 'bad batch'". stuff.co.nz.
- "Archived copy". Archived from the original on 2018-10-18. Retrieved 2018-10-17.CS1 maint: archived copy as title (link)