TCB-2
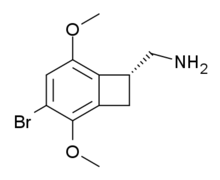
TCB-2 is a hallucinogen discovered in 2006 by Thomas McLean working in the lab of David Nichols at Purdue University where it was named 2C-BCB.[1] It is a conformationally-restricted derivative of the phenethylamine 2C-B, also a hallucinogen, and acts as a potent agonist for the 5-HT2A and 5-HT2C receptors with a Ki of 0.26 nM at the human 5-HT2A receptor. In drug-substitution experiments in rats, TCB-2 was found to be of similar potency to both LSD and Br-DFLY, ranking it among the most potent phenethylamine hallucinogens yet discovered.[1] This high potency and selectivity has made TCB-2 useful for distinguishing 5-HT2A mediated responses from those produced by other similar receptors.[2] TCB-2 has similar but not identical effects in animals to related phenethylamine hallucinogens such as DOI, and has been used for studying how the function of the 5-HT2A receptor differs from that of other serotonin receptors in a number of animal models, such as studies of cocaine addiction and neuropathic pain.[3][4][5][6]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C11H14BrNO2 |
| Molar mass | 272.142 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
See also
References
- McLean, T. H.; Parrish, J. C.; Braden, M. R.; Marona-Lewicka, D; Gallardo-Godoy, A; Nichols, D. E. (2006). "1-Aminomethylbenzocycloalkanes: Conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists". Journal of Medicinal Chemistry. 49 (19): 5794–803. CiteSeerX 10.1.1.688.9849. doi:10.1021/jm060656o. PMID 16970404.
- Chang, C. W.; Poteet, E; Schetz, J. A.; Gümüş, Z. H.; Weinstein, H (2009). "Towards a quantitative representation of the cell signaling mechanisms of hallucinogens: Measurement and mathematical modeling of 5-HT1A and 5-HT2A receptor-mediated ERK1/2 activation". Neuropharmacology. 56 Suppl 1: 213–25. doi:10.1016/j.neuropharm.2008.07.049. PMC 2635340. PMID 18762202.
- Fox, M. A.; French, H. T.; Laporte, J. L.; Blackler, A. R.; Murphy, D. L. (2009). "The serotonin 5-HT2A receptor agonist TCB-2: A behavioral and neurophysiological analysis". Psychopharmacology. 212 (1): 13–23. doi:10.1007/s00213-009-1694-1. PMID 19823806.
- Aira, Z.; Buesa, I.; Salgueiro, M.; Bilbao, J.; Aguilera, L.; Zimmermann, M.; Azkue, J. J. (2010). "Subtype-specific changes in 5-HT receptor-mediated modulation of C fibre-evoked spinal field potentials are triggered by peripheral nerve injury". Neuroscience. 168 (3): 831–841. doi:10.1016/j.neuroscience.2010.04.032. PMID 20412834.
- Katsidoni, V.; Apazoglou, K.; Panagis, G. (2010). "Role of serotonin 5-HT2A and 5-HT2C receptors on brain stimulation reward and the reward-facilitating effect of cocaine". Psychopharmacology. 213 (2–3): 337–354. doi:10.1007/s00213-010-1887-7. PMID 20577718.
- Zhang, G.; Ásgeirsdóttir, H. N.; Cohen, S. J.; Munchow, A. H.; Barrera, M. P.; Stackman, R. W. (2012). "Stimulation of serotonin 2A receptors facilitates consolidation and extinction of fear memory in C57BL/6J mice". Neuropharmacology. 64: 403–13. doi:10.1016/j.neuropharm.2012.06.007. PMC 3477617. PMID 22722027.
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|