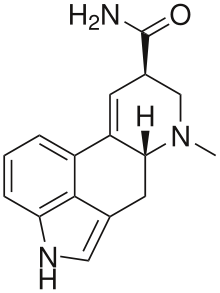
Ergine
Ergine, also known as d-lysergic acid amide (LSA) and d-lysergamide, is an alkaloid of the ergoline family that occurs in various species of vines of the Convolvulaceae and some species of fungi. As the dominant alkaloid in the psychedelic seeds of Turbina corymbosa (ololiuhqui), Argyreia nervosa (Hawaiian baby woodrose) and Ipomoea tricolor (morning glories, tlitliltzin, Badoh negro), it is often stated that ergine and/or isoergine (its epimer) is responsible for the psychedelic activity. However, this theory is debatable, as anecdotal reports suggest that the effects of synthetic LSA and iso-LSA are only slightly psychedelic.[3][4]
 | |
 | |
| Clinical data | |
|---|---|
| Other names | LSA, d-lysergic acid amide, d-lysergamide, Ergine, and LA-111 |
| Pregnancy category |
|
| Routes of administration | Oral, Intramuscular |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | hepatic |
| Excretion | renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.006.841 |
| Chemical and physical data | |
| Formula | C16H17N3O |
| Molar mass | 267.326 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 135 °C (275 °F) Decomposes[2] |
SMILES
| |
InChI
| |
| (verify) | |
History
A traditional use of morning glory seeds by Mexican Native Americans was first described by Richard Schultes in 1941 in a short report documenting their use going back to Aztec times (cited in TiHKAL by Alexander Shulgin). Further research was published in 1960, when Don Thomes MacDougall reported that the seeds of Ipomoea tricolor were used as sacraments by certain Zapotecs, sometimes in conjunction with the seeds of Rivea corymbosa, another species which has a similar chemical composition, with lysergol instead of ergometrine. Ergine was assayed for human activity by Albert Hofmann in self-trials in 1947, well before it was known to be a natural compound. Intramuscular administration of a 500 microgram dose led to a tired, dreamy state, with an inability to maintain clear thoughts. After a short period of sleep the effects were gone, and normal baseline was recovered within five hours.[4]
In 1956, the Central Intelligence Agency conducted research on the psychedelic properties of the ergine in the seeds of Rivea corymbosa, as Subproject 22 of MKULTRA.
Shamanic stories say Ipomoea tricolor and Ipomoea purpurea seeds, when swallowed or chewed, may incite a mild trip where synesthesia occurs and eye imagery is enhanced.[5]
Legal status
There are no laws against possession of ergine-containing seeds in the USA. However, possession of the pure compound without a prescription or DEA license would be prosecuted, as ergine is listed under Schedule III of the Controlled Substances Act.
Occurrence in nature
Ergine has been found in high concentrations of 20 μg/g dry weight in the grass Stipa robusta (sleepygrass) infected with an Acremonium endophytic fungus together with other ergot alkaloids.[6]
Ergine is a component of the alkaloids contained in the Claviceps purpurea (Ergot) fungus which grows on the heads of infected rye grasses.
It is also found in the seeds of several varieties of morning glories in concentrations of approximately 10 μg per seed, as well as Hawaiian baby woodrose seeds, at a concentration of around 0.13% of dry weight.[7]
Known fatalities
There are no known deaths associated directly with pharmacological causes of ergine, but rather due to self-harm, impaired judgement, and drug interactions. One known case involved a suicide that was reported in 1964 after ingestion of morning glory seeds.[8] Another instance is a death due to falling off of a building after ingestion of Hawaiian baby woodrose seeds and alcohol.[9]
See also
- List of entheogenic/hallucinogenic species
- List of psychoactive plants
- Tlitliltzin (Ipomoea violacea)
Notes
- Powell, William (2002). The Anarchist Cookbook. Ozark Press. p. 44. ISBN 978-0-8488-1130-3.
- Smith, Sydney; Timmis, Geoffrey M. (1932). "98. The Alkaloids of Ergot. Part III. Ergine, a New Base obtained by the Degradation of Ergotoxine and Ergotinine". J. Chem. Soc. 1932: 763–766. doi:10.1039/JR9320000763.
References
- Erowid Morning Glory Basics, Erowid.org, retrieved 2012-02-03
- Smith, Sydney; Timmis, Geoffrey Millward (1932). "98. The alkaloids of ergot. Part III. Ergine, a new base obtained by the degradation of ergotoxine and ergotinine". Journal of the Chemical Society (Resumed): 763. doi:10.1039/jr9320000763.
- Perrine, Daniel M. (2000). "Mixing the Kykeon" (PDF). ELEUSIS: Journal of Psychoactive Plants and Compounds. New Series 4: 9.
- Alexander Shulgin, "#26. LSD-25", TiHKAL, Erowid.org, retrieved 2012-02-03
- Erowid Psychoactive Vaults, Erowid.org, retrieved 2012-02-03
- Petroski RJ, Powell RG, Clay K (1992). "Alkaloids of Stipa robusta (sleepygrass) infected with an Acremonium endophyte". Nat. Toxins. 1 (2): 84–88. doi:10.1002/nt.2620010205. PMID 1344912. Archived from the original on 2012-12-16.
- Chao JM, Der Marderosian AH (1973). "Ergoline alkaloidal constituents of Hawaiian baby wood rose, Argyreia nervosa (Burmf) Bojer". J. Pharm. Sci. 62 (4): 588–91. doi:10.1002/jps.2600620409.
- Cohen, Sidney (1964). "SUICIDE FOLLOWING MORNING GLORY SEED INGESTION". The American Journal of Psychiatry. 120 (1): 1024–1025. doi:10.1176/ajp.120.10.1024. PMID 14138842. Retrieved 12 April 2013.
- Klinke, Helene; Irene Breum Müller; Steffen Steffenrud; Rasmus Dahl-Sørensen (15 April 2010). "Two cases of lysergamide intoxication by ingestion of seeds from Hawaiian Baby Woodrose". Forensic Science International. 197 (1): e1–e5. doi:10.1016/j.forsciint.2009.11.017. PMID 20018470.
- Recreational use of D-lysergamide from the seeds of Argyreia nervosa, Ipomoea tricolor, Ipomoea violacea, and Ipomoea purpurea in Poland. Juszczak GR, Swiergiel AH.
- Emergent drugs (III): hallucinogenic plants and mushrooms. [Article in Spanish] Burillo-Putze G, López Briz E, Climent Díaz B, Munné Mas P, Nogue Xarau S, Pinillos MA, Hoffman RS.