Piboserod
Piboserod is a selective 5-HT4 receptor antagonist which was marketed and manufactured by GlaxoSmithKline (GSK) under the trade name Serlipet for the management of atrial fibrillation and irritable bowel syndrome. In 2007 the Norwegian company Bio-Medisinsk Innovasjon AS (BMI)[1] completed a clinical phase II study to investigate the effect of piboserod in patients with chronic heart failure.
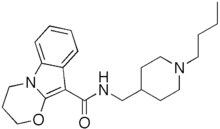 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H31N3O2 |
| Molar mass | 369.50 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Mechanism of action
In 2002 a research group at the University of Oslo discovered that muscles from the ventricle of failing hearts have increased responsiveness to serotonin.[2] They later demonstrated that the effect was due to an expression of functional 5-HT4 receptors in the failing muscle. On the basis of these findings, and in analogy with the success of betablockers in heart failure, the group made the hypothesis that 5-HT4 receptor antagonists could be useful to treat heart failure. Their hypothesis was tested in animal models of heart failure with positive results.[3]
References
- Company web-page
- Bratteli, T.; et al. (2004). "Functional serotonin 5-HT4 receptors in porcine and human ventricular myocardium with increased 5-HT4 mRNA in heart failure. Naunyn-Schmiedeberg's". Naunyn-Schmiedeberg's Archives of Pharmacology. 370 (3): 157–166. doi:10.1007/s00210-004-0963-0. PMID 15365689.
- Birkeland, JA.; et al. (Jan 2007). "Effects of treatment with a 5-HT4 receptor antagonist in heart failure". British Journal of Pharmacology. 150 (2): 143–52. doi:10.1038/sj.bjp.0706966. PMC 2042907. PMID 17160012.