Levoamphetamine
Levoamphetamine[note 1] is a central nervous system (CNS) stimulant known to increase wakefulness and concentration in association with decreased appetite and fatigue. Pharmaceuticals that contain levoamphetamine are currently indicated and prescribed for the treatment of attention deficit hyperactivity disorder (ADHD), obesity, and narcolepsy in some countries.
 | |
| Names | |
|---|---|
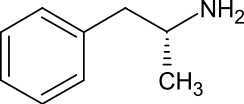
| Systematic IUPAC name
(2R)-1-Phenylpropan-2-amine[1] | |
| Other names
L-Amphetamine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
Beilstein Reference |
2432739 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.320 |
| EC Number |
|
Gmelin Reference |
1125855 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C9H13N |
| Molar mass | 135.2062 g mol−1 |
| log P | 1.789 |
| Pharmacology | |
| Oral (as part of Adderall, Evekeo, and generic amphetamine sulfate[2][3]) | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Levoamphetamine is the levorotatory stereoisomer of the amphetamine molecule.
Chemistry
Levoamphetamine is the levorotatory stereoisomer of the amphetamine molecule. Racemic amphetamine contains two optical isomers, dextroamphetamine and levoamphetamine.[5]
Formulations
Racemic amphetamine
The first patented amphetamine brand, Benzedrine, was a racemic (i.e., equal parts) mixture of the free bases or sulfate salts of both amphetamine enantiomers (levoamphetamine and dextroamphetamine) that was introduced in the United States in 1934 as an inhaler for treating nasal congestion.[2] It was later realized that the amphetamine enantiomers could treat obesity, narcolepsy, and ADHD.[2][3] Because of the greater central nervous system effect of the dextrorotatory enantiomer (i.e., dextroamphetamine), sold as Dexedrine, prescription of the Benzedrine brand fell and was eventually discontinued.[6] However, in 2012 racemic amphetamine sulfate was reintroduced as the Evekeo brandname.[3][7]
Evekeo
Evekeo is an FDA-approved medication that contains racemic amphetamine sulfate (i.e., 50% levoamphetamine sulfate and 50% dextroamphetamine sulfate).[8] It is approved for the treatment of narcolepsy, ADHD, and exogenous obesity.[8]
Others
Products using amphetamine base are now marketed. Dyanavel XR, a liquid suspension form became available in 2015, and contains about 24% levoamphetamine. Adzenys XR, an orally dissolving tablet came to market in 2016 and contains 25% levoamphetamine.[10]
L-Amphetamine succinate was sold in Hungary between 1952 and 1955 under the brand name Cydril.
See also
Notes
- Synonyms and alternate spellings include: (2R)-1-phenylpropan-2-amine (IUPAC name), levamfetamine (International Nonproprietary Name [INN]), (R)-amphetamine, (−)-amphetamine, l-amphetamine, and L-amphetamine.[1][4]
References
- "L-Amphetamine". PubChem Compound. United States National Library of Medicine – National Center for Biotechnology Information. 30 December 2017. Retrieved 2 January 2018.
- Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present – a pharmacological and clinical perspective". J. Psychopharmacol. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
- "Evekeo prescribing information" (PDF). Arbor Pharmaceuticals LLC. April 2014. pp. 1–2. Retrieved 11 August 2015.
- "R(-)amphetamine". IUPHAR/BPS Guide to Pharmacology. International Union of Basic and Clinical Pharmacology. Retrieved 2 January 2018.
- "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. December 2013. Retrieved 30 December 2013.
- "Benzedrine". United States Food and Drug Administration. Retrieved 4 September 2015.
- "Evekeo". United States Food and Drug Administration. Retrieved 11 August 2015.
- "Evekeo Prescribing Information" (PDF). Evekeo (Arbor Pharmaceuticals, LLC). December 2016. Retrieved 6 December 2016.
- "Adzenys XR Prescribing Information" (PDF). United States Food and Drug Administration. Neos Therapeutics, Inc. January 2016. p. 15. Retrieved 7 March 2016.
ADZENYS XR-ODT (amphetamine extended-release orally disintegrating tablet) contains a 3 to 1 ratio of d- to l-amphetamine, a central nervous system stimulant.