Enciprazine
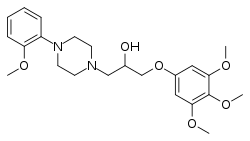
Enciprazine (INN, BAN; enciprazine hydrochloride (USAN); developmental code names WY-48624, D-3112), is an anxiolytic and antipsychotic of the phenylpiperazine class which was never marketed.[1][2][3][4] It shows high affinity for the α1-adrenergic receptor and 5-HT1A receptor, among other sites.[3][5][6] The drug was initially anticipated to produce ortho-methoxyphenylpiperazine (oMeOPP), a serotonin receptor agonist with high affinity for the 5-HT1A receptor, as a significant active metabolite, but subsequent research found this not to be the case.[5]
 | |
| Clinical data | |
|---|---|
| Other names | WY-48624; D-3112 |
| Routes of administration | Oral |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H32N2O6 |
| Molar mass | 432.517 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 485–. ISBN 978-1-4757-2085-3.
- I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 109–. ISBN 978-94-011-4439-1.
- Matheson GK, Knowles A, Gage D, Michel C, Guthrie D, Bauer C, Blackbourne J, Weinzapfel D (1997). "Modification of hypothalamic-pituitary-adrenocortical activity by serotonergic agents in the rat". Pharmacology. 55 (2): 59–65. PMID 9323305.
- "Enciprazine - AdisInsight". adisinsight.springer.com. Retrieved 2017-06-01.
- Scatina JA, Lockhead SR, Cayen MN, Sisenwine SF (1991). "Metabolic disposition of enciprazine, a non-benzodiazepine anxiolytic drug, in rat, dog and man". Xenobiotica. 21 (12): 1591–604. doi:10.3109/00498259109044408. PMID 1686125.
- Linden M, Helmchen H, Müller-Oerlinghausen B (1988). "Early phase-II semi double-blind study of the new alkaline propanolamine derivative enciprazine (short communication)". Arzneimittelforschung. 38 (6): 814–6. PMID 3178922.
External links
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.