Substituted benzofuran
The substituted benzofurans are a class of chemical compounds based on the heterocyclyc and polycyclic compound benzofuran. Many medicines use the benzofuran core as a scaffold,[1][2][3] but most commonly the term is used to refer to the simpler compounds in this class which include numerous psychoactive drugs, including stimulants, psychedelics and empathogens. In general, these compounds have a benzofuran core to which a 2-aminoethyl group is attached (at any position), and combined with a range of other substituents.[4][5][6] Some psychoactive derivatives from this family have been sold under the name Benzofury.[7]

List of substituted benzofurans
The derivatives may be produced by substitutions at six locations of the benzofuran molecule, as well as saturation of the 2,3- double bond.
The following table displays notable derivatives that have been reported:[8][9][10][11][12][13][14][15][16][17][18]
| Structure | Compound | R2 | R3 | R4 | R5 | R6 | R7 | Other modification |
|---|---|---|---|---|---|---|---|---|
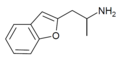 | 2-APB | 2-aminopropyl | H | H | H | H | H | - |
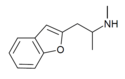 | 2-MAPB | 2-(methylamino)propyl | H | H | H | H | H | - |
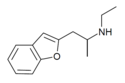 | 2-EAPB | 2-(ethylamino)propyl | H | H | H | H | H | - |
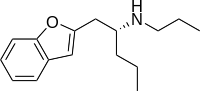 | BPAP | 2-(propylamino)pentyl | H | H | H | H | H | - |
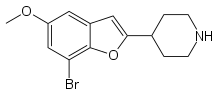 | Brofaromine | 4-piperidinyl | H | H | methoxy | H | bromo | - |
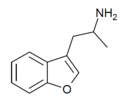 | 3-APB | H | 2-aminopropyl | H | H | H | H | - |
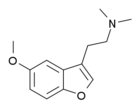 | Dimemebfe | H | 2-(dimethylamino)ethyl | H | methoxy | H | H | - |
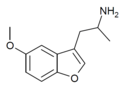 | Mebfap | H | 2-aminopropyl | H | methoxy | H | H | - |
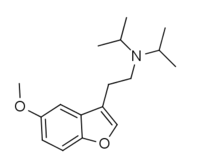 | 5-MeO-DiBF | H | 2-(diisopropylamino)ethyl | H | methoxy | H | H | - |
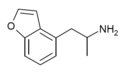 | 4-APB | H | H | 2-aminopropyl | H | H | H | - |
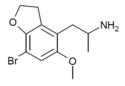 | DOB-5-HEMIFLY (5-MeO-7-Br-4-APDB) | H | H | 2-aminopropyl | methoxy | H | bromo | 2,3-dihydro |
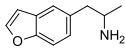 | 5-APB | H | H | H | 2-aminopropyl | H | H | - |
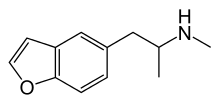 | 5-MAPB | H | H | H | 2-(methylamino)propyl | H | H | - |
| 5-EAPB | H | H | H | 2-(ethylamino)propyl | H | H | - | |
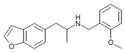 | 5-APB-NBOMe | H | H | H | 2-[(2-methoxybenzyl)amino]propyl | H | H | - |
| 6-APB | H | H | H | H | 2-aminopropyl | H | - | |
 | 6-MAPB | H | H | H | H | 2-(methylamino)propyl | H | - |
| 6-EAPB | H | H | H | H | 2-(ethylamino)propyl | H | - | |
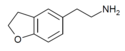 | 5-AEDB | H | H | H | 2-aminoethyl | H | H | 2,3-dihydro |
| 5-APDB | H | H | H | 2-aminopropyl | H | H | 2,3-dihydro | |
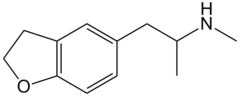 | 5-MAPDB | H | H | H | 2-(methylamino)propyl | H | H | 2,3-dihydro |
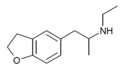 | 5-EAPDB | H | H | H | 2-(ethylamino)propyl | H | H | 2,3-dihydro |
| 6-APDB | H | H | H | H | 2-aminopropyl | H | 2,3-dihydro | |
 | 6-MAPDB | H | H | H | H | 2-(methylamino)propyl | H | 2,3-dihydro |
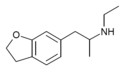 | 6-EAPDB | H | H | H | H | 2-(ethylamino)propyl | H | 2,3-dihydro |
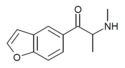 | bk-5-MAPB | H | H | H | 1-oxo-2-(methylamino)propyl | H | H | - |
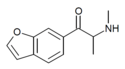 | bk-6-MAPB | H | H | H | H | 1-oxo-2-(methylamino)propyl | H | - |
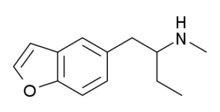 | 5-MBPB | H | H | H | 2-(methylamino)butyl | H | H | - |
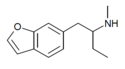 | 6-MBPB | H | H | H | H | 2-(methylamino)butyl | H | - |
 | 5-DBFPV | H | H | H | 1-oxo-2-(pyrrolidin-1-yl)pentyl | H | H | 2,3-dihydro |
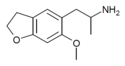 | 6-MeO-5-APDB | H | H | H | 2-aminopropyl | methoxy | H | 2,3-dihydro |
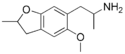 | F-2 | methyl | H | H | methoxy | 2-aminopropyl | H | 2,3-dihydro |
.png) | F-22 | dimethyl | H | H | methoxy | 2-aminopropyl | H | 2,3-dihydro |
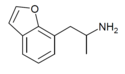 | 7-APB | H | H | H | H | H | 2-aminopropyl | - |
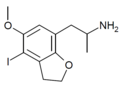 | DOI-2-HEMIFLY (4-I-5-MeO-7-APDB) | H | H | iodo | methoxy | H | 2-aminopropyl | 2,3-dihydro |
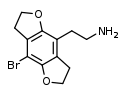 | 2C-B-FLY | H | H | 2-aminoethyl | 5,6-dihydrofuro[5,6-f] | - | bromo | 2,3-dihydro |
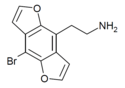 | 2C-B-DRAGONFLY | H | H | 2-aminoethyl | furo[5,6-f] | - | bromo | - |
 | 2C-C-FLY | H | H | 2-aminoethyl | 5,6-dihydrofuro[5,6-f] | - | chloro | 2,3-dihydro |
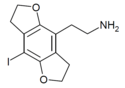 | 2C-I-FLY | H | H | 2-aminoethyl | 5,6-dihydrofuro[5,6-f] | - | iodo | 2,3-dihydro |
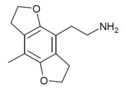 | 2C-D-FLY | H | H | 2-aminoethyl | 5,6-dihydrofuro[5,6-f] | - | methyl | 2,3-dihydro |
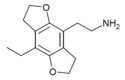 | 2C-E-FLY | H | H | 2-aminoethyl | 5,6-dihydrofuro[5,6-f] | - | ethyl | 2,3-dihydro |
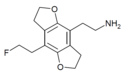 | 2C-EF-FLY | H | H | 2-aminoethyl | 5,6-dihydrofuro[5,6-f] | - | 2-fluoroethyl | 2,3-dihydro |
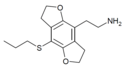 | 2C-T-7-FLY | H | H | 2-aminoethyl | 5,6-dihydrofuro[5,6-f] | - | n-propylthio | 2,3-dihydro |
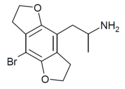 | DOB-FLY | H | H | 2-aminopropyl | 5,6-dihydrofuro[5,6-f] | - | bromo | 2,3-dihydro |
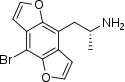 | Bromo-DragonFLY | H | H | 2-aminopropyl | furo[5,6-f] | - | bromo | - |
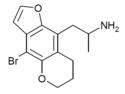 | DOB-2-DRAGONFLY -5-BUTTERFLY |
H | H | 2-aminopropyl | 5,6-dihydropyrano | - | bromo | - |
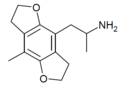 | DOM-FLY | H | H | 2-aminopropyl | 5,6-dihydrofuro[5,6-f] | - | methyl | 2,3-dihydro |
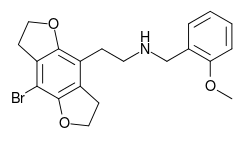 | 2C-B-FLY-NBOMe | H | H | 2-[(2-methoxybenzyl)amino]ethyl | 5,6-dihydrofuro[5,6-f] | - | bromo | 2,3-dihydro |
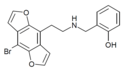 | 2C-B-DRAGONFLY-NBOH | H | H | 2-[(2-hydroxybenzyl)amino]ethyl | furo[5,6-f] | - | bromo | - |
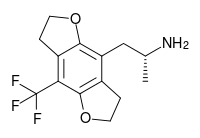 | TFMFly | H | H | 2-aminopropyl | 5,6-dihydrofuro[5,6-f] | - | trifluoromethyl | 2,3-dihydro |
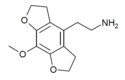 | Mescaline-FLY | H | H | 2-aminoethyl | 5,6-dihydrofuro[5,4-b] | - | methoxy | 2,3-dihydro |
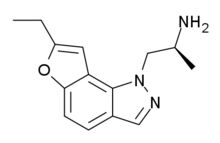 | YM-348 | ethyl | H | 1-(2-aminopropyl)pyrazol[4,5-f] | - | H | H | - |
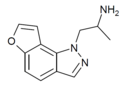 | 2-desethyl-YM-348 | H | H | 1-(2-aminopropyl)pyrazol[4,5-f] | - | H | H | - |
Legislation
Substituted benzofurans saw widespread use as recreational drugs by being sold as research chemicals making them exempt from drug legislation. Many of the more common compounds were banned in the UK on June 2013 as temporary class drugs, while others have been made permanently illegal in various jurisdictions.[19][20][21]
See also
References
- Dawood KM. Benzofuran derivatives: a patent review. Expert Opin Ther Pat. 2013 Sep;23(9):1133-56. doi:10.1517/13543776.2013.801455
- Nevagi RJ, Dighe SN, Dighe SN. Biological and medicinal significance of benzofuran. Eur J Med Chem. 2015 Jun 5;97:561-81. doi:10.1016/j.ejmech.2014.10.085
- Khanam H, Shamsuzzaman. Bioactive Benzofuran derivatives: A review. Eur J Med Chem. 2015 Jun 5;97:483-504. PMID 25482554 doi:10.1016/j.ejmech.2014.11.039
- Nugteren-van Lonkhuyzen JJ, van Riel AJ, Brunt TM, Hondebrink L. Pharmacokinetics, pharmacodynamics and toxicology of new psychoactive substances (NPS): 2C-B, 4-fluoroamphetamine and benzofurans. Drug Alcohol Depend. 2015 Dec 1;157:18-27. PMID 26530501 doi:10.1016/j.drugalcdep.2015.10.011
- Liu C, Jia W, Qian Z, Li T, Hua Z. Identification of five substituted phenethylamine derivatives 5-MAPDB, 5-AEDB, MDMA methylene homolog, 6-Br-MDMA, and 5-APB-NBOMe. Drug Test Anal. 2017 Feb;9(2):199-207. doi:10.1002/dta.1955
- Barcelo B, Gomila I. Pharmacology and Literature Review Based on Related Death and Non-Fatal Case Reports of the Benzofurans and Benzodifurans Designer Drugs. Curr Pharm Des. 2017;23(36):5523-5529. PMID 28714411 doi:10.2174/1381612823666170714155140
- Roque Bravo R, Carmo H, Carvalho F, Bastos ML, Dias da Silva D. Benzo fury: A new trend in the drug misuse scene. J Appl Toxicol. 2019 Aug;39(8):1083-1095. doi:10.1002/jat.3774
- Tomaszewski, Z.; Johnson, M. P.; Huang, X.; Nichols, D. E. (1992). "Benzofuran bioisosteres of hallucinogenic tryptamines". Journal of Medicinal Chemistry. 35 (11): 2061–2064. doi:10.1021/jm00089a017. PMID 1534585.
- Monte AP, Marona-Lewicka D, Cozzi NV, Nichols DE (November 1993). "Synthesis and pharmacological examination of benzofuran, indan, and tetralin analogues of 3,4-(methylenedioxy)amphetamine". Journal of Medicinal Chemistry. 36 (23): 3700–6. doi:10.1021/jm00075a027. PMID 8246240.
- US patent 7045545, Karin Briner, Joseph Paul Burkhart, Timothy Paul Burkholder, Matthew Joseph Fisher, William Harlan Gritton, Daniel Timothy Kohlman, Sidney Xi Liang, Shawn Christopher Miller, Jeffrey Thomas Mullaney, Yao-Chang Xu, Yanping Xu, "Aminoalkylbenzofurans as serotonin (5-HT(2c)) agonists", published 19 January 2000, issued 16 May 2006
- "Temporary class drug order report on 5-6APB and NBOMe compounds". UK Home Office. 4 June 2013. Retrieved 23 August 2016.
- Stanczuk A, Morris N, Gardner EA, Kavanagh P. Identification of (2-aminopropyl)benzofuran (APB) phenyl ring positional isomers in internet purchased products. Drug Test Anal. 2013 Apr;5(4):270-6. doi: 10.1002/dta.1451. PMID 23349125
- Nichols DE, Hoffman AJ, Oberlender RA, Riggs RM. Synthesis and evaluation of 2,3-dihydrobenzofuran analogues of the hallucinogen 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane: drug discrimination studies in rats. J Med Chem. 1986 Feb;29(2):302-4. PMID 3950910
- Nichols DE, Snyder SE, Oberlender R, Johnson MP, Huang XM. 2,3-Dihydrobenzofuran analogues of hallucinogenic phenethylamines. J Med Chem 1991; 34:276–281. DOI: 10.1021/jm00105a043
- Monte AP, Marona-Lewicka D, Parker MA, Wainscott DB, Nelson DL, Nichols DE. Dihydrobenzofuran analogues of hallucinogens. 3. Models of 4-substituted (2,5-dimethoxyphenyl)alkylamine derivatives with rigidified methoxy groups. J Med Chem 1996, 39:2953–2961. DOI: 10.1021/jm960199j
- Liu C, Jia W, Qian Z, Li T, Hua Z. Identification of five substituted phenethylamine derivatives 5-MAPDB, 5-AEDB, MDMA methylene homolog, 6-Br-MDMA, and 5-APB-NBOMe: Identification of five substituted phenethylamine derivatives. Drug Test Analysis, 1 Jan 2016. doi: 10.1002/dta.1955
- Wagmann L, Brandt SD, Stratford A, Maurer HH, Meyer MR. Interactions of phenethylamine-derived psychoactive substances of the 2C-series with human monoamine oxidases. Drug Test Anal. 2019 Feb;11(2):318-324. doi:10.1002/dta.2494
- Wagmann L, Hempel N, Richter LHJ, Brandt SD, Stratford A, Meyer MR. Phenethylamine-derived new psychoactive substances 2C-E-FLY, 2C-EF-FLY, and 2C-T-7-FLY: Investigations on their metabolic fate including isoenzyme activities and their toxicological detectability in urine screenings. Drug Test Anal. 2019 Jul 12. doi:10.1002/dta.2675
- UK Home Office (5 March 2014). "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". UK Government. Retrieved 23 August 2016.
- "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 23 August 2016.
- "指定薬物名称・構造式一覧(平成27年9月16日現在)" (PDF) (in Japanese). 厚生労働省. 16 September 2015. Retrieved 23 August 2016.