Vilanterol
Vilanterol (INN, USAN) is an ultra-long-acting β2 adrenoreceptor agonist (ultra-LABA), which was approved in May 2013 in combination with fluticasone furoate for sale as Breo Ellipta by GlaxoSmithKline for the treatment of chronic obstructive pulmonary disease (COPD).[1]
 | |
| Clinical data | |
|---|---|
| License data | |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.217.751 |
| Chemical and physical data | |
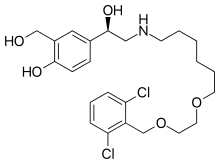
| Formula | C24H33Cl2NO5 |
| Molar mass | 486.43 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Vilanterol is available in following combinations:
- with inhaled corticosteroid fluticasone furoate—fluticasone furoate/vilanterol (trade names Breo Ellipta (U.S.), Relvar Ellipta (EU, RU, JPN))
- with muscarinic antagonist umeclidinium bromide—umeclidinium bromide/vilanterol (trade name Anoro Ellipta)
- with inhaled corticosteroid fluticasone furoate and muscarinic antagonist umeclidinium bromide—fluticasone furoate/umeclidinium bromide/vilanterol (trade name Trelegy Ellipta)
See also
- Salmeterol—a long-acting β2 adrenoreceptor agonist (LABA) with a similar backbone.
References
- "FDA approves Breo Ellipta to treat chronic obstructive pulmonary disease". Retrieved 10 May 2013.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.