Abediterol
Abediterol (INN;[2] development codes AZD-0548 and LAS 100977) is a once-daily experimental drug candidate for the treatment of asthma and chronic obstructive pulmonary disease (COPD). It is currently under development by the Spanish pharmaceutical company Almirall and is in Phase II clinical trials.[3][4]
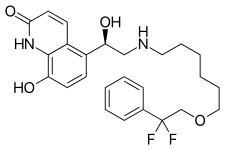 | |
| Clinical data | |
|---|---|
| Routes of administration | Inhalation |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 24.3 hours[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H30F2N2O4 |
| Molar mass | 460.522 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
It acts as a dual β2 adrenergic agonist[5][6] and muscarinic antagonist and is classified as an ultra-long-acting β2 agonist (ultra-LABA).[7]
Its coformulation with mometasone furoate is also in Phase II clinical trials.[8]
References
- Timmer, Wolfgang; Massana, Eric; Jimenez, Eulalia; Seoane, Beatriz; de Miquel, Gonzalo; Ruiz, Sandrine (December 2014). "First-in-Human Study of the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Abediterol (LAS100977), a Novel Long-Acting β2-agonist". The Journal of Clinical Pharmacology. 54 (12): 1347–53. doi:10.1002/jcph.355.
- "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed International Nonproprietary Names: List 104" (PDF). WHO Drug Information. 24 (1): 352. 2010. Retrieved 25 March 2016.
- Product development pipeline, Almirall
- "AdisInsight: Abediterol". Adis Insight. Springer International Publishing AG. Retrieved 25 March 2016.
- WO 2006122788
- WO 2010094484
- Beier, J; Fuhr, R; Massana, E; Jiménez, E; Seoane, B; de Miquel, G; Ruiz, S (October 2014). "Abediterol (LAS100977), a novel long-acting β2-agonist: Efficacy, safety and tolerability in persistent asthma". Respiratory Medicine. 108 (10): 1424–1429. doi:10.1016/j.rmed.2014.08.005. PMID 25256258. Retrieved 25 March 2016.
- "AdisInsight: Abediterol/mometasone". Adis Insight. Springer International Publishing AG. Retrieved 25 March 2016.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.